Consider the process which is carried out at constant pressure. The total S for this process is
Question:
Consider the process
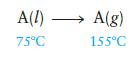
which is carried out at constant pressure. The total △S for this process is known to be 75.0 J K21 mol21. For A(l) and A(g), the Cp values are 75.0 J K21 mol21 and 29.0 J K21 mol21, respectively, and are not dependent on temperature. Calculate △H vaporization for A(l) at 1258C.
Transcribed Image Text:
A(l) →→→ A(g) 75°C 155°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the process which is carried out at constant pressure. The total DS for this process is known to be 75.0 J K-1mol-1. For A(l) and A(g), the Cp values are 75.0 JK-1mol-1 and 29.0 JK-1mol-1,...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
According to the Australian Department of Industry, Tourism, and Resources (DITR), 8.6% of the total employment in NSW is related to manufactured exports. A sample of 110 employees in NSW is randomly...
-
In Exercises 8194, begin by graphing the absolute value function, f(x) = |x|. Then use transformations of this graph to graph the given function. h(x) = x + 4 - 2
-
Arguing about economics late one night in your dorm room, your friend says, In a free market economy, if people are willing to pay a lot for something, then businesses will charge a lot for it. One...
-
A stock price is currently $30. During each two-month period for the next four months it is expected to increase by 8% or decrease by 10%. No dividend payment is expected during these two periods....
-
What are three ways in which the production, development and use of IT are causing unethical behavior?
-
The financial statements of Victors Natural Foods include the following items: .:. Requirement 1. Compute the following ratios for the current year: a. Current ratio b. Acid-test ratio c. Inventory...
-
Evaluate the following definite integral. 1 x (4x 1)4 dz 0 1/3 X
-
Prepaid Expense On December 15, Company B paid $18,000 for a six-month insurance policy starting on that day. a. How much should Company B show as Insurance Expense for the year ended December 31?...
-
High temperatures are favorable to a reaction kinetically but may be unfavorable to a reaction thermodynamically. Explain.
-
It has been determined that the body can generate 5500 kJ of energy during one hour of strenuous exercise. Perspiration is the bodys mechanism for eliminating this heat. What mass of water would have...
-
Solve the rational inequality 5 4 + 2x V 0
-
What is the role of natural buyers and natural sellers in order-driven markets?
-
Why should an investor who believes that the market is efficient pursue an indexing strategy?
-
How do order-driven and quote-driven market structures differ?
-
What are the three general types of stock market indicators?
-
The November 1985 prospectus of the Merrill Lynch Phoenix Fund, Inc., a mutual fund, stated the following invesiment objective: Based upon the belief that the pricing mechanism of the securities...
-
A manufacturing concern's total cost function is C = X/5 and its revenue function is R = 39 log(8X + 1). Find the optimum output where the profit is maximized and the maximum profit.
-
Describe the Operations (+,,*,/) that can cause negligible addition (NA), error magnification (EM), or subtractive cancellation (SC) in calculating ?((x^2)+1) - x . Give the range of where they might...
-
Acetic acid is responsible for the sour taste of vinegar. It can be manufactured using the following reaction: Use tabulated values of bond energies (Table 13.6) to estimate DH for this reaction....
-
Use bond energies (Table 6), values of electron affinities (Table 8), and the ionization energy of hydrogen (1312 kJ/ mol) to estimate DH for each of the following reactions. a. HF(g) H+(g) + F-(g)...
-
The standard enthalpies of formation of S(g), F(g), SF4(g), and SF6(g) are 1278.8 kJ/ mol, 179.0 kJ/ mol, 775 kJ/ mol, and 1209 kJ/ mol, respectively. a. Use these data to estimate the energy of an...
-
The Step Company has the following Information for the year just ended: Sales in units Budget 15,000 Actual 14,000 Sales $150,000 $147,000 Less: Variable Expenses 90,000 82,600 Contribution Margin $...
-
Question 2 (2 marks) The year level coordinators for Years 7 to 11 at the school are Amy (A), Brian (B), Claire (C), Daisy (D) and Ellie (E). A faulty telephone system means that some of these...
-
On a certain farm alfalfa yields have been 4.3, 7.2, 5.6, and 3.6 tons per acre in the last 4 years. What is the expected value for the farm's alfalfa yield, if each past result is given equal weight...

Study smarter with the SolutionInn App


