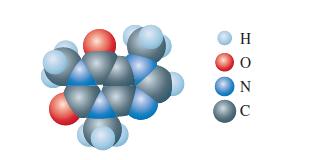
Consider your Lewis structure for the computer-generated model of caffeine shown in Exercise 108 of Chapter 13.
Question:
Consider your Lewis structure for the computer-generated model of caffeine shown in Exercise 108 of Chapter 13. How many \(\mathrm{C}\) and \(\mathrm{N}\) atoms are \(s p^{2}\) hybridized in your Lewis structure for caffeine? How many \(\mathrm{C}\) and \(\mathrm{N}\) atoms are \(s p^{3}\) hybridized? \(s p\) hybridized? How many \(\sigma\) and \(\pi\) bonds are in your Lewis structure?
Lewis structure of Exercise 108 of Chapter 13
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





