Diborane (B 2 H 6 ) is a highly reactive boron hydride that was once considered as
Question:
Diborane (B2H6) is a highly reactive boron hydride that was once considered as a possible rocket fuel for the U.S. space program. Calculate ΔH for the synthesis of diborane from its elements, according to the equation
![]()
using the following data:
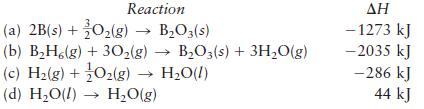
Transcribed Image Text:
2B(s) + 3H(g) - BH6(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To obtain AH for the required reaction we must somehow com bine equations a b c and d to produce tha...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The formation of tetrafluoroethylene from its elements is highly exothermic: (a) If a mixture of F2, graphite, and C2F4 is at equilibrium in a closed container, will the reaction go to the right or...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Propane, C 3 H 8 , is a gas used as a fuel for outdoor grills and alternative-fuel vehicles. The enthalpy change for the synthesis of propane from its elements in their standard states is difficult...
-
Label the following as True, False, or Uncertain and explain your choice. (Uncertain means that it can be either true or false depending upon the circumstances.) a. All members of a resource cartel...
-
The parameters F = 0.7 and c = 0.05 are fixed for Equation 4.43 describing the driven, damped pendulum. Determine which of the values for w (0.1, 0.2, 0.3.., 1.5) produce chaotic motion. Produce a...
-
Explain how to change the minimum cardinality when a parent that was required to have a child is no longer required to have one.
-
Fun-Tastic Shows, Inc., is a company that hosts carnivals and similar events. Susan Swartwood, Crystal Groth, and a minor (named in the case as M.G.S.) attended Fun-Tastics Rhododendron Festival in...
-
Joses Electronic Repair Shop has budgeted the following time and material for 2014. Joses budgets 5,000 hours of repair time in 2014 and will bill a profit of $5 per labor hour along with a 30%...
-
Sales for a new car is expected to grow according to the equation: S = 150000(1-e -0.06t ), where t = months i) Calculate the number of cars sold after one year. ii) Calculate the number of cars sold...
-
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and...
-
It has been suggested that hydrogen gas obtained from the decomposition of water might be a substitute for natural gas (principally methane). To compare the energies of combustion of these fuels, the...
-
No ducks waltz. No officers ever decline to waltz. All my poultry are ducks. My poultry are not officers. The following sorites are taken from Lewis Carrolls Symbolic Logic. All are valid. Rewrite...
-
This is the first step in developing a risk assessment and management policy. In developing your policy, consideration of risk appetite is critical. Identify the areas of financial and operational...
-
The balance sheet and income statement provide essential information to prepare the cash flow statement. The relationship between these two financial reports with the statement of cash flows may be...
-
Wee Works specializes in renting space to very small high tech start ups. It owns several properties in various cities in the US that are trying to attract start ups. Even though it is a bit outside...
-
The annual budget for Vasconcellos Manufactures for 2023 is the following: Direct Materials......$30,000 Direct Labor ($8 per hour; yeah, I'm cheap!)........120,000 Sales Commissions.....28,000...
-
From the reading above as well as other sources define all of the following at the level of a senior in a top University, and commit them to permanent memory: Capital, Capitalism, Money, Profit,...
-
When is the basic cost flow model used? Give an example.
-
Refer to Exercise 8.S.I. Construct a scatterplot of the data. Does the appearance of the scatterplot indicate that the pairing was effective? Explain. Exercise 8.S.I. A volunteer working at an animal...
-
Explain why K + ions move more rapidly through water than Li + ions.
-
Suggest a reason why the size of the silicon atom does not permit a silicon analog of the graphite structure.
-
Write a balanced chemical equation for the industrial preparation of impure boron.
-
Which is the definitio Process of partcipating firsthand in the communication process as a listener, rather than just passively listening to what is said. Process of hearing what others say with the...
-
What two characteristics do group / team techniques have in common?
-
What kind of view of entrepreneurship does this theory support? What does this theory suggest about those who may not want to start a business? What does it say about the innate desires of such...

Study smarter with the SolutionInn App


