During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of
Question:
During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of the acid was found to have a pH of 10.35. Use Table 6C.1 to identify the acid.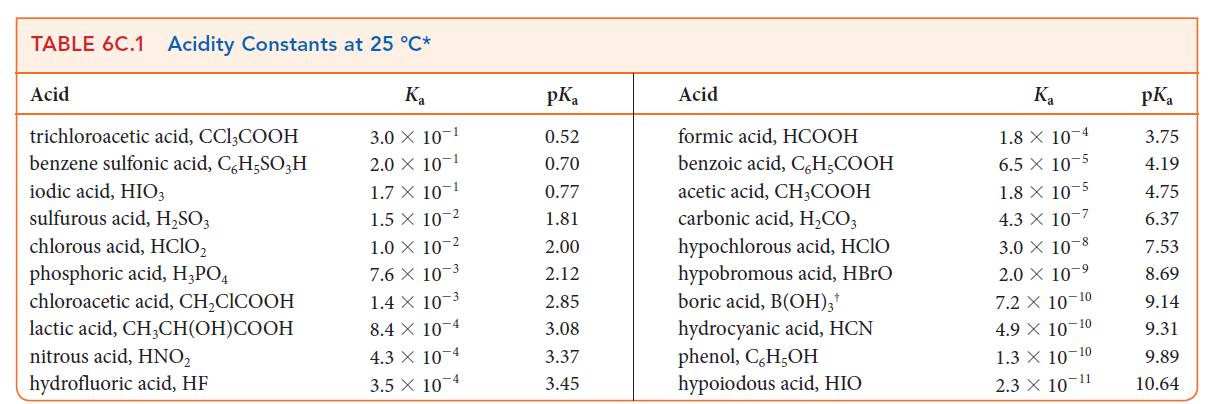
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* K₂ 3.0 X 10-1 2.0 X 10-1 1.7 X 10-1 1.5 X 10-2 1.0 × 10-2 7.6 X 10-3 1.4 x 10-3 8.4 X 10-4 4.3 X 10-4 3.5 x 10-4 Acid trichloroacetic acid, CC13COOH benzene sulfonic acid, C,H,SO3H iodic acid, HIO3 sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H3PO4 chloroacetic acid, CH₂CICOOH lactic acid, CH₂CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF pK₂ 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H,COOH acetic acid, CH₂COOH carbonic acid, H₂CO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3¹ hydrocyanic acid, HCN phenol, C,H,OH hypoio dous acid, HIO K₂ 1.8 X 10 4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 X 10-8 2.0 × 10-9 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 × 10 11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The fo...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
During the analysis of an unknown weak base B, a 0.10 m solution of the nitrate salt of the base was found to have a pH of 3.13. Use Table 6C.2 to identify the base. TABLE 6C.2 Basicity Constants at...
-
The partnership agreement of Axel, Berg & Cobb provides for the year-end allocation of net income in the following order: First, Axel is to receive 10% of net income up to $100,000 and 20% over...
-
Can we use servomotor for position control? Support the answer with necessary details
-
A manufacturer claims that the average tensile strength of thread A exceeds the average tensile strength of thread B by at least 12 kilograms. To test his claim, 50 pieces of each type of thread are...
-
If a loop of chain is spun at high speed, it will roll like a hoop without collapsing. Consider a chain of linear mass density m that is rolling without slipping at a high speed v 0 . (a) Show that...
-
Analyze the competitive structure of the automobile industry in the United States. Is this an attractive industry? As the first decade of the 21st century drew to a close, the global automobile...
-
In a large corporate computer network, user log-ons to the system can be modeled as a Poisson process with a mean of 25 log-ons per hour. What is the probability that there are no log-ons in an...
-
1. Based on the information in this case, provide examples, for Siemens, of at least four strategically required organizational outcomes, and four required workforce competencies and behaviors. 2....
-
Our new client, Laser Company, is being audited for the first time on December 3I of Year 3, the end of its accounting period. In the course of our examination, we encounter in the ledger an assel...
-
Aritzia Inc.s financial statements have been reproduced in Appendix A at the back of the textbook. Instructions a. Many companies use a calendar year for their financial statements. What does Aritzia...
-
Calculate the molar concentration of H 3 O + in solutions with the following molar concentrations of OH : (a)0.0021 mol L 1 ; (b) 3.4 * 10 3 mol L 1 ; (c)7.60 mmol L 1
-
Decide which acid in each of the following pairs is the stronger and explain why: (a) H 3 AsO 4 or H 3 PO 4 ; (b) HBrO 3 or HBrO; (c) H 3 PO 4 or H 3 PO 3 ; (d) H 2 Te or H 2 Se; (e) H 2 S or HCl;...
-
If the resultant of the four forces is F R = {?360k} lb, determine the tension developed in each cable. Due to symmetry, the tension in the four cables is the same. Fc 6 ft 3it
-
A company owns a 6-year-old gear hobber that has a book value of \($60,000.\) The present market value of the hobber is \($80,000.Anew\) gear hobber can be purchased for \($450,000.\) Using an...
-
Dell is considering replacing one of its material handling systems. The old system was purchased 7 years ago for \($130,000\) and was depreciated as MACRS-GDS 5-year property since the system is used...
-
A company owns a 5-year-old turret lathe that has a book value of $20,000. The present market value of the lathe is $16,000. A new turret lathe can be purchased for $45,000. Using a before-tax...
-
Allen Construction purchased a crane 6 years ago for \($130,000.\) They need a crane of this capacity for the next 5 years. Normal operation costs \($35,000\) per year. The current crane will have no...
-
Clear Water Company has a down-hole well auger that was purchased 3 years ago for \($30,000.\) O&M costs are \($13,000\) per year. Alternative A is to keep the existing auger, which has a current...
-
According to data released by the World Bank, the mean PM10 (particulate matter) concentration for the city of Kabul, Afghanistan, in 1999 was 46. Suppose that because of efforts to improve air...
-
Michelles trust is subject to 3.8% surtax on the lesser of the trusts net investment income or the excess of the trusts adjusted gross income over the $12,400 threshold (the highest trust tax rate)....
-
Many processes such as the fabrication of integrated circuits are carried out in a vacuum chamber to avoid reaction of the material with oxygen in the atmosphere. It is difficult to routinely lower...
-
A 3.50 mole sample of N 2 in a state defined by T i = 250.K and V i = 3.25 L undergoes an isothermal reversible expansion until V f = 35.5 L. Calculate w assuming a. That the gas is described by the...
-
A major league pitcher throws a baseball with a speed of 162 kilometers per hour. If the baseball weighs 235 grams and its heat capacity is 1.7 J g 1 K 1 , calculate the temperature rise of the ball...
-
Let -2 5 -5 4 5 2 -2 -10 A = and b = 5 -3 2 -7 2 -1 -4 11 Define the linear transformation T : R3 R4 by T(x) = Ax. Find a vector x whose image under T is b. Is the vector x unique? choose
-
In The fifth Discipline, Senge (1990) advises that in a learning organization the leader's activities are different than a traditional organization and focus on designing, and continually refining,...
-
PERFORMANCE TASK IN PCOM CREATE AN ARGUMENTATIVE ESSAY ABOUT: (MAKE A CATCHY TITLE ABOUT YOUR ESSAY) IS ANIMAL TESTING MORAL? In making argumentative essay Make sure to: 1. Make a claim 2. Develop...

Study smarter with the SolutionInn App


