For the enzyme-catalyzed conversion of a certain substrate, K M = 0.038 mol L 1 at
Question:
For the enzyme-catalyzed conversion of a certain substrate, KM = 0.038 mol · L–1 at 25°C. When the substrate concentration is 0.156 mol · L–1, the rate of the reaction is 1.21 mmol · L–1s–1. The maximum rate of the conversion reaction is reached at high substrate concentrations (see Exercise 7.14). Calculate the maximum rate of this enzyme-catalyzed reaction.
Exercise 7.14
From the following mechanism, derive Eq. 1a in Topic 7E, which Michaelis and Menten proposed to represent the rate of formation of products in an enzyme-catalyzed reaction.
Show that the rate is independent of substrate concentration at high concentrations of substrate.
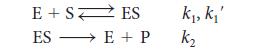
where E is the free enzyme, S is the substrate, ES is the enzyme– substrate complex, and P is the product. Note that the steady-state concentration of free enzyme will be equal to the initial concentration of the enzyme less the amount of enzyme that is present in the enzyme–substrate complex: [E] = [E]0 = [ES].
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





