For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: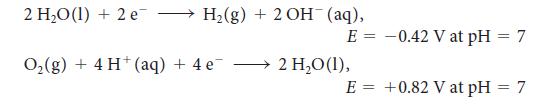
(a) When a current of 324 mA is used for 15 h, what volume (measured in liters at 298 K and 1.0 atm) of fluorine gas can be produced from a molten mixture of potassium and hydrogen fluorides?
(b) With the same current and time period, how many liters of oxygen gas at 298 K and 1.0 atm can be produced from the electrolysis of water?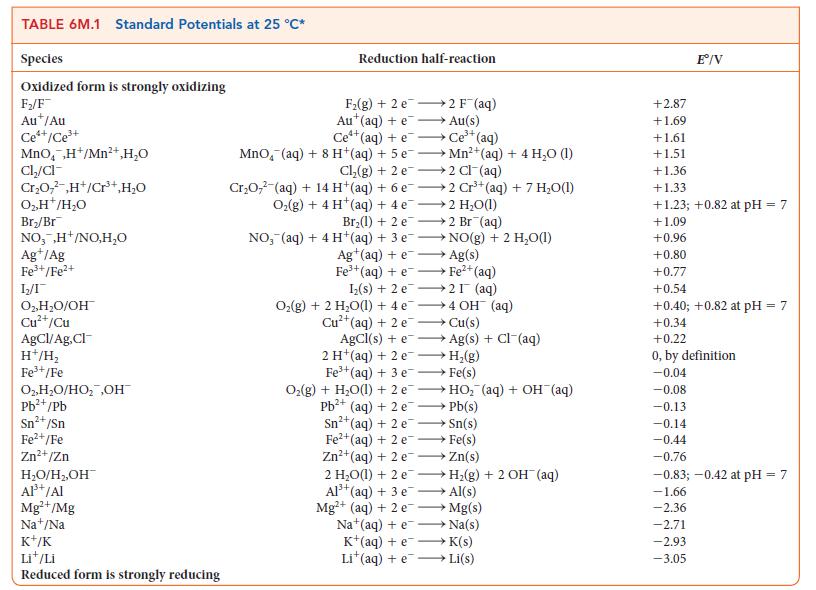
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





