In an experiment, 0.100 mol SO 3 was introduced into a flask of volume 2.00 L and
Question:
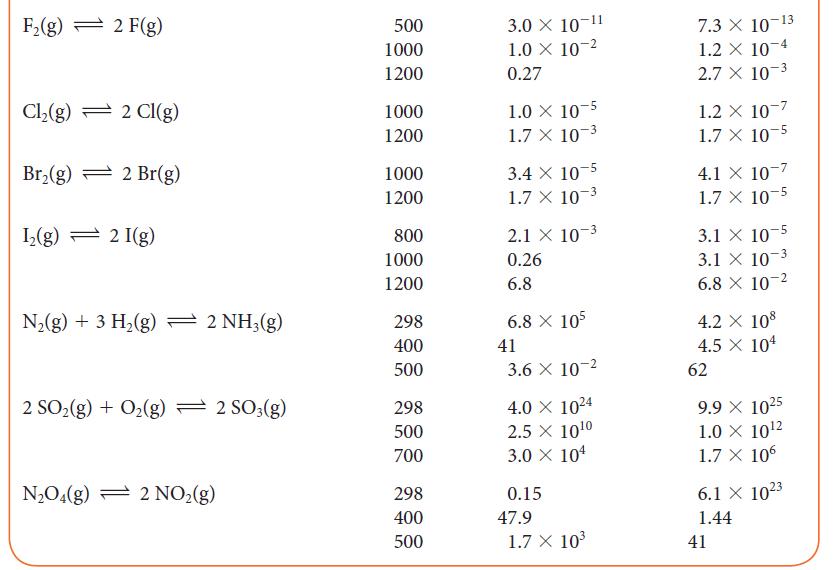
In an experiment, 0.100 mol SO3 was introduced into a flask of volume 2.00 L and the reaction 2 SO2(g) + O2(g) ⇌ 2 SO3(g) was allowed to come to equilibrium at 700 K.
(a) Using information in Table 5G.2, calculate the equilibrium concentrations of the three gases.
(b) The volume of the flask is increased to 6.00 L. Calculate the new equilibrium concentrations of the gases.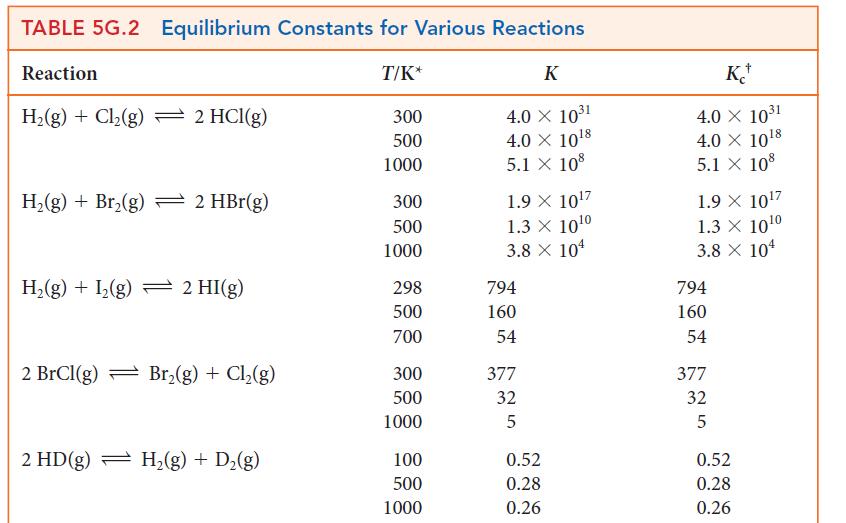
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





