Investigators are studying the physical properties of a gas to be used as a refrigerant in an
Question:
Investigators are studying the physical properties of a gas to be used as a refrigerant in an air-conditioning unit. Because refrigerants are used at low temperature and high pressure, they cannot be treated as ideal gases. A table of van der Waals parameters shows that for a certain refrigerant a = 16.4 bar · L2 · mol–2 and b = 8.4 * 10–2 L·mol–1. Estimate the pressure of the gas when 1.50 mol occupies 5.00 L at 0 °C.
ANTICIPATE Fig. 3E.1 suggests that in most gases under normal conditions, the attractive interaction dominates the repulsive. So, you should suspect that the calculated pressure will be less than that based on an ideal gas, but the difference will be small, because real gases typically show only small deviations from ideal behavior.
PLAN Substitute the data into Eq. 4 after converting the temperature into the Kelvin scale. Use R in units that match those given.
FIGURE 3E.1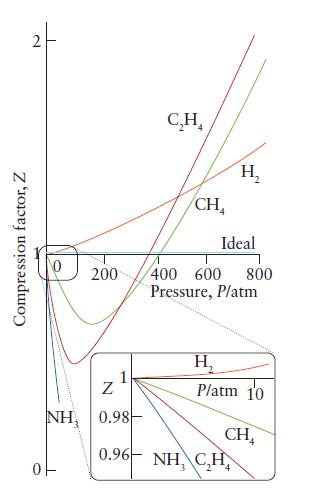
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





