Mean bond enthalpies can be used to estimate the enthalpy of reaction when precise data are not
Question:
Mean bond enthalpies can be used to estimate the enthalpy of reaction when precise data are not available. Estimate the enthalpy of the reaction between liquid bromine and gaseous propene to form liquid 1,2-dibromopropane. The enthalpy of vaporization of Br2 is 29.96 kJ · mol–1, and that of CH3CHBrCH2Br is 35.61 kJ · mol–1. The reaction is![]()
ANTICIPATE Two bonds must break in the reactants (Br—Br and C=C), whereas three bonds form in the product (C—C and two C—Br). Because more bonds are forming than are breaking, you should anticipate that the reaction is likely to be exothermic. Energy is required to vaporize the bromine, but it may be balanced by the energy released when the product condenses. However, the C=C bond has a high bond enthalpy, so you should expect the reaction to be only mildly exothermic.
PLAN Decide which bonds are broken and which bonds are formed. Use the mean bond enthalpies in Table 4E.3 to estimate the change in enthalpy when the reactant bonds break and the change in enthalpy when the new product bonds form.
For diatomic molecules, use the information in Table 4E.2 for the specific molecule. Then, add the enthalpy change required to break the reactant bonds (a positive value) to the enthalpy change that occurs when the product bonds form (a negative value). Finally, because bond enthalpies are for gaseous substances, include the appropriate enthalpies of vaporization.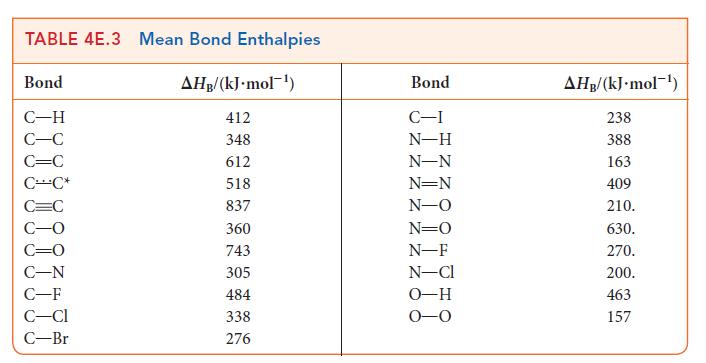
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





