One of the skills of a scientist is to be able to verify mathematically what is understood
Question:
One of the skills of a scientist is to be able to verify mathematically what is understood conceptually.
For example, does the increase in entropy of the surroundings account for the fact that a liquid freezes when the temperature is lowered to below a certain temperature?
Calculate the change in entropy of the surroundings per mole of Hg when mercury freezes at 249°C; use ΔHfus(Hg) = 2.292 kJ · mol–1 at 249°C.
ANTICIPATE The normal freezing point of mercury is 238°C, so you should certainly expect it to freeze at a lower temperature. A contribution to this spontaneous freezing is the release of energy as heat to the surroundings, causing their entropy to increase (FIG. 4I.3).
PLAN To calculate the change in entropy of the surroundings when mercury freezes, write ΔHfreeze = 2ΔHfus(freezing is the opposite of fusion) and convert the temperature to kelvins.
The change in entropy of the system, the mercury itself, is 29.782 J · mol–1.
What should you assume? Assume that the temperature of the surroundings is constant.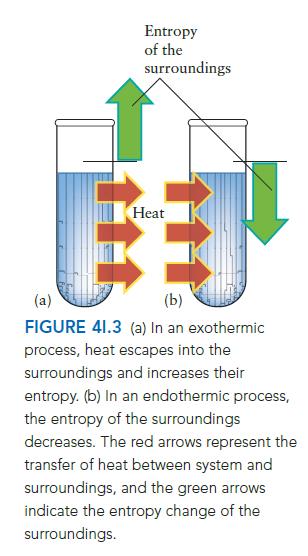
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





