Petroleum-based fuels contribute to climate change, and alternative fuels are being sought (see Box 4D.1). Three compounds
Question:
Petroleum-based fuels contribute to climate change, and alternative fuels are being sought (see Box 4D.1). Three compounds that could be produced biologically and used as fuels are methane, CH4, which can be produced from the anaerobic digestion of sewage; dimethyl ether, H3C—O—CH3, a gas that can be produced from methanol and ethanol; and ethanol, CH3CH2OH, a liquid obtained from the fermentation of sugars.
(a) Draw the Lewis structure of each compound.
(b) Use bond enthalpies (and, for ethanol, its enthalpy of vaporization) to calculate the enthalpy of combustion of each fuel, assuming that they burn to produce gaseous CO2 and gaseous H2O. Explain any differences.
(c) Use the values for the enthalpies of combustion of organic compounds found in Appendix 2A to compare methane and ethanol with octane, a primary constituent of gasoline, as fuels by calculating the specific enthalpy (heat produced per gram) of each fuel. On the basis of this information, which would you choose as a fuel?
(d) What volume of methane gas at 10.00 atm and 298 K would you need to burn at constant pressure to produce the same amount of heat as 10.00 L of octane (the density of octane is 0.70 g·mL–1)?
(e) A problem with fuels containing carbon is that they produce carbon dioxide when they burn, and so a consideration governing the selection of a fuel could be the heat per mole of CO2 produced. Calculate this quantity for methane, ethanol, and octane. Which process produces more carbon dioxide in the environment for each kilojoule generated?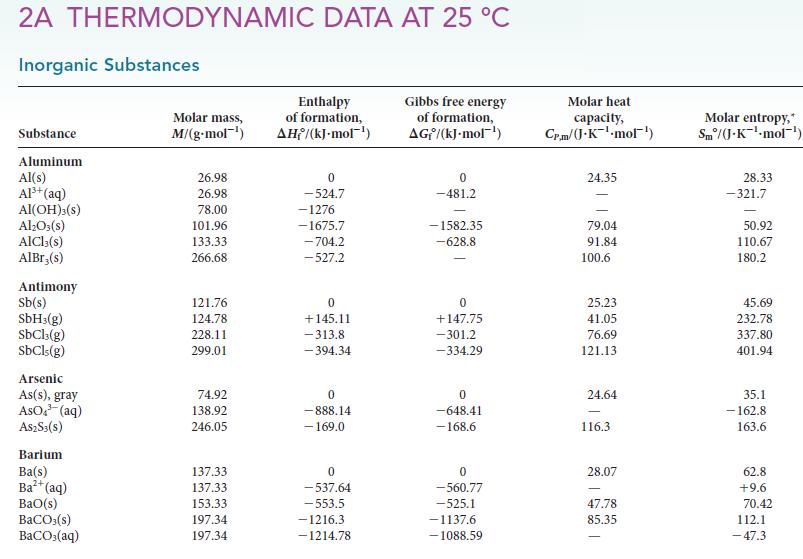
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





