Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase:
Question:
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: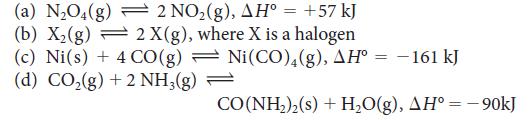
Transcribed Image Text:
2 NO₂(g), AH° = +57 kJ 2 X(g), where X is a halogen → (a) N₂O4(g) (b) X₂(g) (c) Ni(s) + 4CO(g) (d) CO₂(g) + 2 NH3(g) Ni(CO)4(g), AH = -161 kJ CO(NH₂)₂(s) + H₂O(g), AH° = - 90kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a and b are endothermic and raising ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: (a) CH4(g) + HO(g) (b) CO(g) + HO(g) (c) 2 SO(g) + O(g) CO(g) +3 H(g), AH = +206...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Predict whether each of the following nuclides is stable or unstable (radioactive). If the nuclide is unstable, predict rhe type of radioactivity you would expect it to exhibit. a. 4519K b. 5626Fe c....
-
Factor each polynomial. 125k - 64k 4
-
The space between two metallic coaxial cylinders of length L and radii a and b is completely filled with a material having a resistivity . (a) What is the resistance between the two cylinders? (b)...
-
What factors should be considered when selecting a site for a new manufacturing facility?
-
Analyze the Frank-Kamenetskii problem for the three standard geometries of slab, cylinder, and sphere. You will need to discretize the operators suitably for the cylinder and sphere. Plot the...
-
Consider an experiment with four groups, with eight values in each. For the ANOVA summary table below, fill in all the missing results: Mean Degrees of Sum of Square Source Freedom Squares (Variance)...
-
More info The company manufactures a variety of engines for use in farm equipment. At the beginning of the current year, Dansville estimated that its overhead for the coming year would be $300,000....
-
Suppose you have a list of blood platelet counts from 500 patients in a hospital. Which of the following is most helpful in understanding the distribution of those values: frequency table, pie chart,...
-
(a) Calculate the mass of CaCl 2 6H 2 O needed to prepare 0.125 m CaCl 2 (aq) by using 500. g of water. (b) What mass of NiSO 4 6H 2 O must be dissolved in 500. g of water to produce 0.22 m NiSO 4...
-
Write the reaction quotient Q for (a) 2 BCl3(g) + 2 Hg(1) BCl(s) + HgCl(s) (b) P4S10(s) + 16 HO(1) 4 H3PO4(aq) + 10 HS(aq) (c) Br(g) + 3 F(g) 2 BrF3(g)
-
In Exercise 36, does it seem possible that the population mean could equal half the sample mean? Explain. Data from Exercise 36: In a random sample of 18 months from June 2008 through September 2016,...
-
Describe some of the threats to external validity that are common with the survey approach.
-
Define the two major types of sampling strategies .
-
What type of scenario would warrant the application of a longitudinal design overall a cross-sectional design?
-
Identify a hypothetical population .
-
How would you describe the consumer identity of the Beyhive the Beyonce fans? Are there particular personality or lifestyle characteristics that members of the Beyhive share?
-
Determine the outstanding principal balance on the loan in Problem 12 after 20 payments have been made. In Problem 12, Determine the monthly payment for a sixty-month truck loan with an annual...
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
Is the following statement correct? Because dry ice sublimes, carbon dioxide has no liquid phase. Explain your answer.
-
For the equation of state V m = RT /P + B(T), show that ' () = -T- d?
-
The following data are a DSC scan of a solution of a T4 lysozyme mutant. From the data determine T m . Determine also the excess heat capacity ÎC P at T = 308 K. Determine also the intrinsic...
-
Blossom Company applies manufacturing overhead to jobs on the basis of machine hours used. Overhead costs are estimated to total $ 3 7 2 , 0 0 0 for the year, and machine usage is estimated at 1 5 5...
-
Convert the following number from base 10 to binary (base 2): 92 Fill in the blanks to get the correct result. 92 modulo 2 11 0 46 modulo 2 0 23 modulo 2 = 1 11 modulo 2 = 1 Blank #1 modulo 2 = Blank...
-
Convert the following number from base 10 to binary (base 2). 50 Fill in the blanks to get the correct result. 50 25 12 6 Blank #1 Blank #3 modulo 2 II 0 modulo 2 = 1 modulo 2 = 0 modulo 2 = 0 modulo...

Study smarter with the SolutionInn App


