Question:
Stratospheric ozone, O3, protects life on Earth from harmful ultraviolet radiation from the Sun. Suppose you are an atmospheric chemist; to understand the spectroscopic and structural properties of ozone, you would need to know how its electrons are arranged. Suggest two Lewis structures that contribute equally to the resonance structure for the O3 molecule. Experimental data show that the two bond lengths are the same.
ANTICIPATE You should expect to be able to write structures that differ only in the position of a multiple bond.
PLAN Write a Lewis structure for the molecule by using the method outlined in Toolbox 2B.1. Decide whether there is another equivalent structure that results from the interchange of a single bond and a double or triple bond. Write the actual structure as a resonance hybrid of these Lewis structures.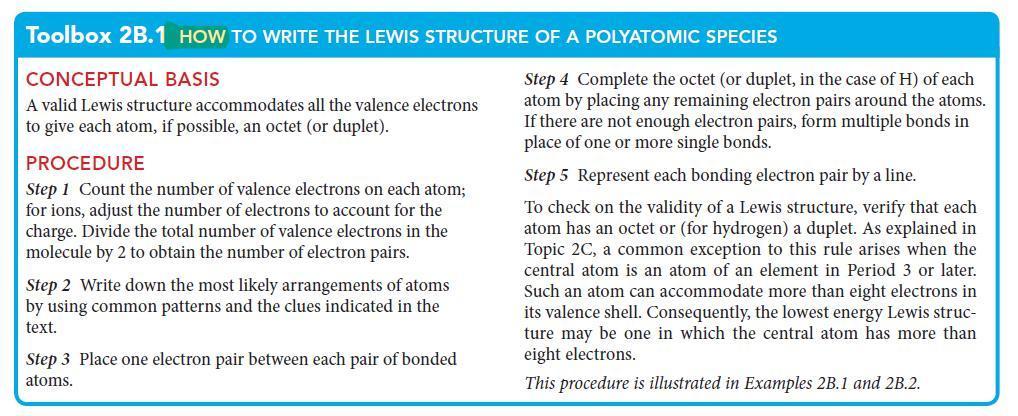
Transcribed Image Text:
Toolbox 2B.1 HOW TO WRITE THE LEWIS STRUCTURE OF A POLYATOMIC SPECIES
CONCEPTUAL BASIS
A valid Lewis structure accommodates all the valence electrons
to give each atom, if possible, an octet (or duplet).
PROCEDURE
Step 1 Count the number of valence electrons on each atom;
for ions, adjust the number of electrons to account for the
charge. Divide the total number of valence electrons in the
molecule by 2 to obtain the number of electron pairs.
Step 2 Write down the most likely arrangements of atoms
by using common patterns and the clues indicated in the
text.
Step 3 Place one electron pair between each pair of bonded
atoms.
Step 4 Complete the octet (or duplet, in the case of H) of each
atom by placing any remaining electron pairs around the atoms.
If there are not enough electron pairs, form multiple bonds in
place of one or more single bonds.
Step 5 Represent each bonding electron pair by a line.
To check on the validity of a Lewis structure, verify that each
atom has an octet or (for hydrogen) a duplet. As explained in
Topic 2C, a common exception to this rule arises when the
central atom is an atom of an element in Period 3 or later.
Such an atom can accommodate more than eight electrons in
its valence shell. Consequently, the lowest energy Lewis struc-
ture may be one in which the central atom has more than
eight electrons.
This procedure is illustrated in Examples 2B.1 and 2B.2.







