Suppose you are designing a chemical plant that is providing phosphorus compounds to other industries and you
Question:
Suppose you are designing a chemical plant that is providing phosphorus compounds to other industries and you need to explore the equilibrium properties for the reaction of PCl5(g) ⇌ PCl3(g) + Cl2(g). The reaction has reached equilibrium at 250 °C (the equilibrium partial pressures of the components are PPCl5 = 0.02 bar, PPCl3 = 1.28 bar, PCl2 = 1.28 bar, and K = 78.3). You add 0.0100 mol Cl2(g) to the equilibrium mixture in the container (of volume 500. mL); then the system is once again allowed to reach equilibrium. Use this information to calculate the new composition of the equilibrium mixture.
ANTICIPATE Because a product has been added, you should expect the reaction to respond by forming reactants at the expense of products.
PLAN The general procedure is like that set out in Toolbox 5I.1, except that the direction of reaction might not be toward products. Write the expression for the equilibrium constant, and then set up an equilibrium table. In this case, use as the initial partial pressures those found immediately after addition of the reagent but before the reaction has responded.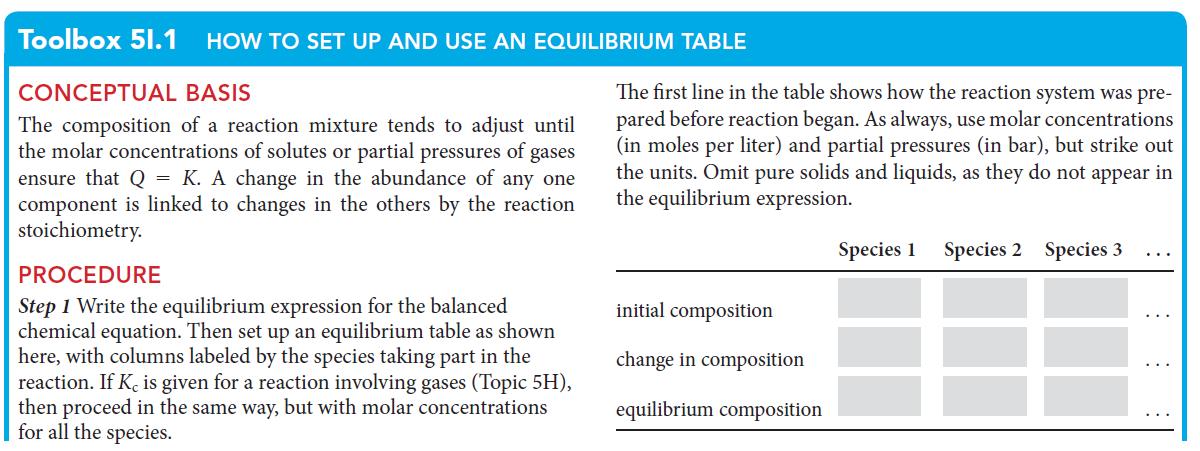
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





