The following plot shows a system composed of the gaseous compounds A and B in a rigid,
Question:
The following plot shows a system composed of the gaseous compounds A and B in a rigid, constant-volume flask. The system was initially at equilibrium, then a change occurred.
(a) Describe the change that occurred and how it affected the system.
(b) Write the chemical equation for the reaction that occurred.
(c) Calculate the value of Kc for that reaction.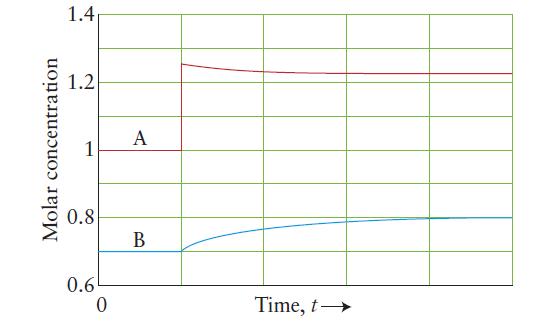
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





