The following rate data were collected for the reaction 2 A(g) + 2 B(g) + C(g)
Question:
The following rate data were collected for the reaction 2 A(g) + 2 B(g) + C(g) → 3 G(g) + 4 F(g):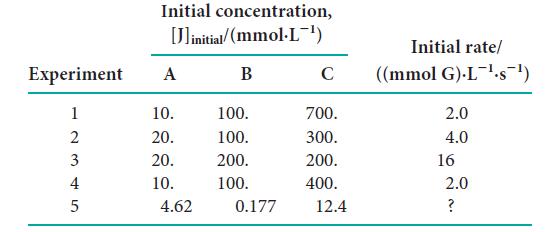 (a) What is the order for each reactant and the overall order of the reaction?
(a) What is the order for each reactant and the overall order of the reaction?
(b) Write the rate law for the reaction.
(c) Determine the reaction rate constant.
(d) Predict the initial rate for Experiment 5.
Transcribed Image Text:
Initial concentration, [linitial/(mmol.L-¹) B Experiment A 12345 10. 20. 20. 10. 4.62 100. 100. 200. 100. 0.177 C 700. 300. 200. 400. 12.4 Initial rate/ ((mmol G).L¹-s¯¹) 2.0 4.0 16 2.0 ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a first order with respect to ...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1933+ Reviews
4269+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The outer and inner wheels are spun. Assuming that the wheels are independent and the outcomes are equally likely, determine the probability of obtaining. Numbers greater than 5 on both wheels. 3 2 4...
-
Use the pattern below. Each figure is made of squares that are 1 unit by 1 unit. a. Find the distance around each figure. Organize your results in a table. b. Use your table to describe a pattern in...
-
In Exercises delete part of the domain so that the function that remains is one-to-one. Find the inverse function of the remaining function and give the domain of the inverse function. f(x) = (x - 3)...
-
Determine the degrees of freedom under the following conditions: (a) Tl-20 wt% Pb at 325 C and 400 C; (b) Tl-40 wt% Pb at 325 C and 400 C; (c) Tl-90 wt% Pb at 325 C and 400 C. Refer to the phase...
-
Here is a bogus About me description. Which of the following passwords could be guessed from the About Me? a. Walker b. jebBrother c. IBeat Al d. 1600Penn-Ave.NW e. texas
-
The countershaft in Prob. 372, p. 138, is part of a speed reducing compound gear train using 20 spur gears. A gear on the input shaft drives gear A. Gear B drives a gear on the output shaft. The...
-
What is the definition of ignorable treatment assignment? a. Give an example of a study where the treatment assignment is ignorable. b. Give an example of a study where the treatment assignment is...
-
Golden State Enterprises provides consulting services throughout California and uses a job-order costing system to accumulate the cost of client projects. Traceable costs are charged directly to...
-
Selected data from the statement of cash flows of Park Corporation are as follows: Operating Activities Net cash provided by (used in) operating activities Investing Activities $ 46,000 Additions to...
-
Sulfuryl chloride, SO 2 Cl 2 , decomposes by first-order kinetics, and k r = 2.81 * 10 3 min 1 at a certain temperature. (a) Determine the half-life for the reaction. (b) Determine the time needed...
-
Repeat Exercise 7C.13 for the same reaction taken to be second order in each direction. Exercise 7C.13 Consider the reaction A B, which is first order in each direction with rate constants k r and k...
-
What is minority interest and where is it reported on the consolidated balance sheet?
-
A chemical company has acquired a site for their new plant. They required to enclose that field with a fence. They have 700 meter of fencing material with a building on one side of the field where...
-
On your own or with a classmate, choose one of the following goods or services. Decide whether you want to market it as a consumer product or a business product. Now create a brand name to convey the...
-
Classify each of the following business-to-consumer (B2C) and business-to-business (B2B) goods and services. Then choose one and describe how it could be classified as both. a. Runner's World or...
-
A $100 \mathrm{~kg}$ man is preparing to spend the day on a dive. If we can approximate the man as a cylinder, $2 \mathrm{~m}$ long and $0.2 \mathrm{~m}$ in radius, how much weight must he carry to...
-
A steel cable holds a $250 \mathrm{~kg}$ tank below the surface of saltwater. If the volume of water displaced by the tank is $0.2 \mathrm{~m}^{3}$, what is the tension in the cable? Assume the...
-
Are top executives paid too much? Do the companies run by the most highly paid executives perform better than other companies? Do U.S. executives make more money than their foreign counterparts?...
-
Consider a game of poker being played with a standard 52-card deck (four suits, each of which has 13 different denominations of cards). At a certain point in the game, six cards have been exposed. Of...
-
You drew the two chair conformations of lindane. Carefully inspect them, and predict the difference in energy between them, if any.
-
Compound A exists predominantly in a chair conformation, while compound B exists predominantly in a twist boat conformation. Explain. Compound A Compound B
-
Draw Haworth projections for cis-1,3-dimethylcyclohexane and trans-1,3-dimethylcyclohexane. Then, for each compound, draw the two chair conformations. Use these conformations to determine whether the...
-
Boom and bust are equally likely and occur with probability . Hillton currently has 100 of debt outstanding, due next period, which is backed by the combined cash flow of the two divisions. (a) What...
-
Return on investment, or ROI is a commonly used profitability ratio that measures the amount of return an investment generates relative to its costs.What are some limitations to ROI?
-
Someone offers to sell you an annuity for $6,036 in 5 years (i.e., you will buy it at t=5). The annuity pays $2300 per year, for n years, starting in year 6, and we assume a 7.0% discount rate. About...

Study smarter with the SolutionInn App


