The following table lists the van der Waals b parameters for Br 2 , N 2 ,
Question:
The following table lists the van der Waals b parameters for Br2, N2, He, and SF6. Without referring to Table 3E.1, use your knowledge of the factors governing the magnitudes of b to assign each of these four gases to its b value. Explain your reasoning.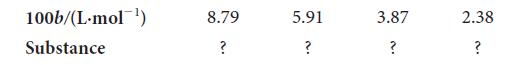
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





