The height, h, of a column of liquid in a capillary tube can be estimated by using
Question:
The height, h, of a column of liquid in a capillary tube can be estimated by using h = 2γ/gdr, where γ is the surface tension, d is the density of the liquid, g is the acceleration of free fall (g = 9.806 m · s–2), and r is the radius of the tube. Which will rise higher in a tube that is 0.15 mm in diameter at 25°C, water or ethanol? The density of water is 0.997 g · cm–3 and that of ethanol is 0.79 g· cm–3. See Table 3G.1.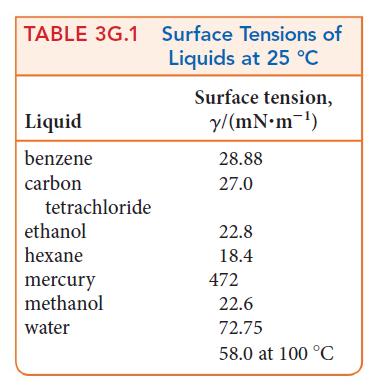
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





