The reaction [2 mathrm{NO}(g)+mathrm{Cl}_{2}(g) longrightarrow 2 mathrm{NOCl}(g)] was studied at (-10^{circ} mathrm{C}). The following results were obtained,
Question:
The reaction
\[2 \mathrm{NO}(g)+\mathrm{Cl}_{2}(g) \longrightarrow 2 \mathrm{NOCl}(g)\]
was studied at \(-10^{\circ} \mathrm{C}\). The following results were obtained, where
\[\text { Rate }=-\frac{d\left[\mathrm{Cl}_{2}ight]}{d t}\]
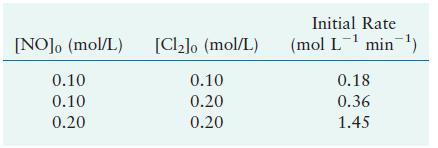
a. What is the rate law?
b. What is the value of the rate constant?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





