There are three isomers of difluoroethene, C 2 H 2 F 2 , which differ in the
Question:
There are three isomers of difluoroethene, C2H2F2, which differ in the locations of the fluorine atoms.
(a) Which of the forms are polar?
(b) Which has the largest dipole moment?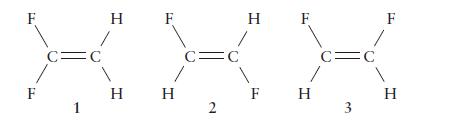
Transcribed Image Text:
F F C= 1 C O H F H H C= 2 H F F H C= 3 F H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Polarity Isomer 1 transdifluoroethene Nonpolar In this isomer the CF bond dipoles cancel each othe...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
There are three isomers of dichlorobenzene, C 6 H 4 Cl 2 , which differ in the relative positions of the chlorine atoms on the benzene ring. (a) Which of the three forms are polar? (b) Which has the...
-
A student claims there are three isomers of propanol: 1-propanol, 2-propanol, and 3-propanol. Is he correct? Explain.
-
A molecule XF6 (having no lone pairs) has a dipole moment of zero. (X denotes an unidentified element.) When two atoms of fluorine have been taken away, you get the molecule XF4, which has dipole...
-
Consider the following independent situations found during audit testing of Faran Ltd, which has a balance date of 30 June 2019. Assume that all the situations are material. (i)Recent industrial...
-
Why should the factors under the control of the investment center manager (revenues, expenses, and invested assets) be considered in computing the rate of return on investment?
-
Name and briefly describe one category of usage-based market segmentation.
-
Why does an exchange difference arise and how it is recognised under the temporal method of translation used to translate financial statements of foreign operations?
-
The May 2012 revenue and cost information for Houston Outfitters, Inc., follows: Requirement 1. Prepare a standard cost income statement for management through gross profit. Report all standard cost...
-
a) Explain the following terms as used in real estate appraisals i. Value in use ii. iii. Liquidation value Insurable value b) Describe the cost approach of real estate valuation c) Explain the...
-
Draw Lewis structures for each of the following species and predict the hybridization at each carbon atom: (a) H 2 CCH ; (b) H 2 CCH 3 + ; (c) H 3 CCH 2 .
-
Explain why the lattice energy of lithium chloride (861 kJ mol -1 ) is greater than that of rubidium chloride (695 kJ mol -1 ), given that they have similar arrangements of ions in the crystal...
-
A floral arrangement consists of 6 different colored roses, 3 different colored carnations, and 3 different colored daisies. You can choose from 8 different colors of roses, 6 different colors of...
-
Controversy surrounded the three-factor model due to skepticism by researchers. Black (1993, 1995) said that the model had two problems. Discuss these two potential problems.
-
Did Fama and French (1996) find evidence to support a link between earnings and the size and value factors? Review their findings. What are the underlying state variables that produce variation in...
-
Kothari, Shanken, and Sloan (1995) found evidence to support the CAPM. Also, they criticized the Fama and French studies. Review these issues in their study.
-
Kolari, Liu, and Huang (KLH) (2021) argued that the mean-variance parabola is shaped by the dual systematic risk effects of beta risk and zeta risk. How is the architecture of the parabola affected...
-
Fama and French (1996) argued that size and value factors are linked to economic fundamentals such as earnings within the firm. What were their arguments in this regard?
-
Suppose that an increase in world tensions makes it more likely than before that there will be a nuclear war within 10 years. Such a war would kill half the worlds population and destroy 90% of the...
-
Refer to the Conservation Ecology (Dec. 2003) study of the causes of forest fragmentation, presented in Exercise 2.166 (p. 97). Recall that the researchers used advanced high-resolution satellite...
-
The decomposition of N 2 O 5 in the gas phase was studied at constant temperature: The following results were collected: Using these data, verify that the rate law is first order in [N 2 O 5 ], and...
-
The reaction between bromate ions and bromide ions in acidic aqueous solution is given by the following equation: Table 15.5 gives the results of four experiments involving this reaction. Using these...
-
The compound \(\mathrm{NO}_{2} \mathrm{Cl}\) is thought to decompose to \(\mathrm{NO}_{2}\) and \(\mathrm{Cl}_{2}\) by the following mechanism: Derive the rate law for the production of...
-
Dr. Solo is preparing a single journal entry for December 31, 2022. The bank statement shows a balance of $10,500 on that day. Three checks were made out on that day: one for $250 for medical...
-
Zoe deposited $10,000 in her savings account on March 1, 2023. The savings account earns her 3.5%. a) What will be balance of Zoe's savings account on November 1, 2023? b) How much interest did Zoe...
-
Williams company purchased a truck for 38000 with an expected life of five years and residual value of 3800 at the end of the year three the truck has accumulated depreciation of 20520 six months...

Study smarter with the SolutionInn App


