To analyze the alcohol content of a certain wine, a chemist needs 1.00 L of an aqueous
Question:
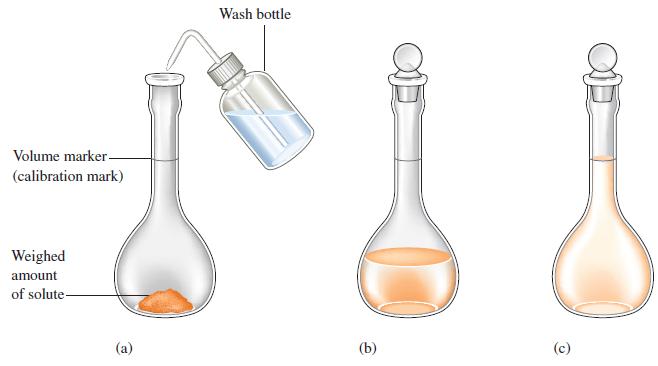
To analyze the alcohol content of a certain wine, a chemist needs 1.00 L of an aqueous 0.200 M K2Cr2O7 (potassium dichromate) solution. How much solid K2Cr2O7 must be weighed out to make this solution?
Figure 4.9
Transcribed Image Text:
Volume marker- (calibration mark) Weighed amount of solute- (a) Wash bottle (b) (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
First determine the moles of KCrO7 required 0200 mol KCrO7 L solution 100 L ...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
The energy content of a certain food is to be determined in a bomb calorimeter that contains 3 kg of water by burning a 2-g sample of it in the presence of 100 g of air in the reaction chamber. If...
-
What is the is the effect of abolition of an import quota on (i) national saving, (ii) domestic investment, (iii) NCO, (iv) the real exchange rate, and (v) net exports?
-
A rod is bent into a circular arc of radius 4 in. as shown. For the given loading, determine the internal forces at point J when 30 θ = °. 12 Ih B. 4 in.
-
Prove Theorem 10.7. The solution to the equation T(N) = aT(N/b) + (Nk logp N), where a 1, b > 1, and p 0 is O(Nlog a) if a > bk | T(N) = {O(N* logP+1 N) if a = b* O(N* log? N) if a < bk
-
Presented here are the accounts of Plantscapes Decor Services Corporation for the year ended December 31, 2011. Requirements 1. Prepare Plantscapes Decor Services Corporations income statement. 2....
-
A: Suppose a firm employs labor and capital k to produce output x using a homothetic, decreasing returns to scale technology. (a) Suppose that, at the current wage w, rental rate r and output price...
-
Question 38 (5 points) 25.00 Expected Return (%) 20.00+ 15.00- 10.00 5.00 F 0.00 0 10 20 Gold 30 D Optimal CAL Standard Deviation (%) Portfolios B and D in the graph above lie outside the efficient...
-
Using the solubility rules in Table 4.1, predict what will happen when the following pairs of solutions are mixed. a. KNO 3 (aq) and BaCl 2 (aq) b. Na 2 SO 4 (aq) and Pb(NO 3 )2(aq) c. KOH(aq) and...
-
Typical blood serum is about 0.14 M NaCl. What volume of blood contains 1.0 mg of NaCl?
-
The following are the financial statements for Nailsea plc for the years ended 30 June 2020 and 2021 There were no disposals of non-current assets in either year. Dividends were paid in 2020 and 2021...
-
Why is compassion and empathy valuable traits to have as a leader ?
-
Calculate the \Delta H of the Target Reaction:, 2C2H4O(1) + 2H2O(1)2C2H6O(l) +0(g) Step Reactions: Eqn. 1C,H60(1) + 302(g) 2C02(g) + 3H20(1), AH = 685kJ Eqn. 2C, HO(l) +0(g) 2CO(g) + 2H2O(l),AH =...
-
What is coercive power? Why is it not considered a form of leadership? What is the difference between socialized and personalized charismatic leaders? Explain the concept of competitive advantage and...
-
Describe the shape and factors that affect the demand curve and the supply curve in a market. Discuss the concept of market equilibrium and how changes in demand and supply affect the equilibrium...
-
Impact of current global financial crisis on CIMB Group. -use the annual report as a reference -look at articles to elaborate
-
Fill in the blanks in the schedule below for two separate investment centers A and B. Round answers to the nearest wholepercent. Investment Center $10.400,000 $.400,000 Average invested assets Profit...
-
The company manufactures three products: wooden chairs, tables and dressers. AFC started off as a 'Mom & Pop' shop but has grown rapidly. AFC uses one assembly line to build all three products,...
-
What is supersaturation? Under what conditions is it possible to supersaturate a solution? What is the metastable region?
-
Does the commonly reported solubility of an inorganic compound in water pertain to large crystals or small crystals? Why?
-
Can an inorganic compound have more than one form of hydrate?
-
Currently, ACME Inc. has a capital structure consisting of 35% debt and 65% equity. ACME's debt currently has a 8% yield to maturity. The risk-free rate is 4%, and the market risk premium is 6%....
-
Earley Corporation issued perpetual preferred stock with an 7% annual dividend. The stock currently yields 9%, and its par value is $100. What is the preferred stock's value?
-
A pension fund manager is considering three mutual funds. The first is a stock fund, the second is a long-term government and corporate bond fund, and the third is a T-bill money market fund that...

Study smarter with the SolutionInn App


