Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) +
Question:
Use the data in Appendix 2B and the fact that, for the half-reaction F2(g) + 2 H+(aq) + 2 e– → 2 HF(aq), E° = 13.03 V, to calculate the value of Ka for HF.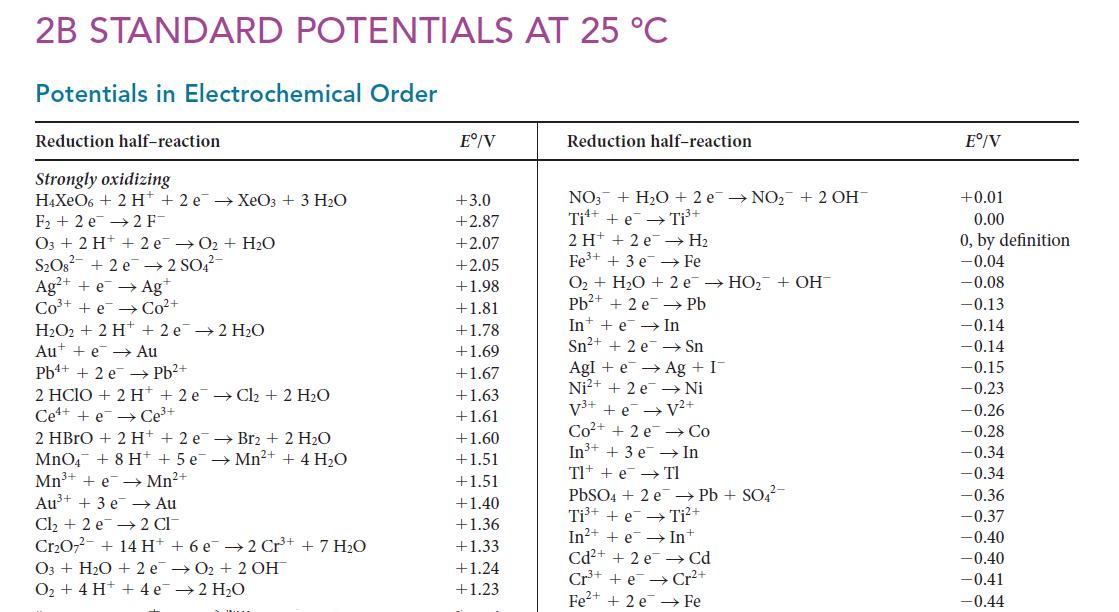
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6+ 2 H+2 e → XeO3 + 3 H₂O F₂2 e 2 F- O3 + 2 H+ 2 e→O₂ + H₂O S₂O8² +2e →2 SO4²- Ag²+ +e → Agt Co³+ + e Co²+ H₂O2 + 2 H Au + e→→ Pb+ + 2 e + 2e →2 H₂O Au Pb²+ 2 HClO + 2 H+2 e → Cl₂ + 2 H₂O Cee Ce³+ →Mn²+ + 4H₂O 2 HBrO + 2 H+2 e Br2 + 2 H₂O MnO4 + 8 H+ + 5 e Mn³+ + e→ Mn²+ Au³+ + 3 e → Au Cl₂ + 2 e 2 CI Cr₂O7²- + 14 H+ + 6 e 2 Cr³+ + 7 H₂O O3+ H₂O + 2e →O₂ + 2 OH → 2H₂O O₂ + 4H+ + 4e Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 Reduction half-reaction NO3 + H₂O + 2e →NO₂+ 2 OH Ti + e Ti³+ 2H+ +2 e Fe³+ + 3 e → H₂ Fe O₂ + H₂O + 2e → HO₂ + OH Pb²+ + 2 e pb In e In Sn²+ + 2 e AgI+ e → Sn Ag + I → Ni Ni²+ + 2e V³+ + e → V²+ Co²+ + 2e In³+ + 3 e Tl +e →→ Tl → Co In PbSO4 + 2 e Pb + SO4²- Ti³+ + e Ti²+ In²++eIn+ Cd²+ + 2 e Cd Cr³+ + eCr²+ Fe²+ + 2 e Fe Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
2...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2B to calculate E(Ti 3+ /Ti). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 HO F +2e...
-
Use the data in Appendix 2B to calculate E(U 4+ /U). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F+2 e...
-
Use only the data in Appendix 2B to calculate the acidity constant of HClO in water. 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing...
-
Compute the stone's average velocity over the time interval [0.5, 2.5] and indicate the corresponding secant line on a sketch of the graph of h(t). A stone is tossed vertically into the air from...
-
ROI and RI. (D. Kleespie, adapted) The Outdoor Sports Company produces a wide variety of outdoor sports equipment. Its newest division, Golf Technology, manufactures and sells a single product:...
-
On March 31, Dower Publishing discounted a $30,000 note at a local bank. The note was dated February 28 and required the payment of the principal amount and interest at 6% on May 31. The bank's...
-
A heat pipe is an evaporator-condenser system in which the liquid is returned to the evaporator by capillary action. In the simplest form it consists of a wire-mesh region that serves to act as a...
-
Mario, age 65, purchased an immediate annuity for $120,000 that pays a lifetime monthly income of $1000. The annuity has no refund feature. Based on the IRS actuarial table, Mario has a life...
-
Oslo Company prepared the following contribution format income statement based on a sales volume of 1,000 units (the relevant range of production is 500 units to 1,500 units): Sales Variable expenses...
-
Suppose that 25.0 mL of a solution of Ag + ions of unknown concentration is titrated with 0.015 m KI(aq) at 25C. A silver electrode is immersed in this solution, and its potential is measured...
-
Dental amalgam, a solid solution of silver and tin in mercury, was used for filling tooth cavities. Two of the reduction halfreactions that the filling can undergo are Suggest a reason why, if you...
-
A selected set of organizations may run a blockchain node separately for keeping the transaction records. Administrators from the organizations establish the access rights and permissions for each...
-
Why is an independent review of work accomplishment preferred over having the foreman responsible for the work do the assessment?
-
What are the three main considerations involved in managing subcontractors?
-
What criteria are considered in deciding on the frequency of progress reporting for a project?
-
What are the four planning quality characteristics that will likely improve the reliability of planning at the activity level?
-
To improve overall production through the application of lean principles, where does the Last Planner Process $\odot$ focus?
-
Public companies that operate in different businesses or segments are required to report operating results for those segments. This requirement makes it possible to evaluate the performance of...
-
Evenflow Power Co. is considering a new project that is a little riskier than the current operations of the company. Thus, management has decided to add an additional 1.5% to the company's overall...
-
The maximum theoretical efficiency of an internal combustion engine is achieved in a reversible Carnot cycle. Assume that the engine is operating in the Otto cycle and that C V ,m = 5/2 R for the...
-
The following two compounds each exhibit two heteroatoms (one nitrogen atom and one oxygen atom). In compound A, the lone pair on the nitrogen atom is more likely to function as a base. However, in...
-
2.25 moles of an ideal gas with C V ,m = 5/2 R is transformed from an initial state T 680. K and P = 1.15 bar to a final state T = 298.K and P = 4.75 bar. Calculate U, H, and S for this process.
-
10t) The sum of signal x(t) = sin(40 10 t) and AWG noise n(t) is fed to a filter H(f) whose spectral characteristic is shown in the figure. Input noise power density is No-10-6 [W/Hz] 1 |H(f)\ 18...
-
A typical telephone channel has the bandwidth of 3100 Hz can support dial-up modems that can variable speeds depending on the quality of the links on both ends of the data call. a) (4 points)...
-
6. Consider the following algorithm. Give a function with one term and coefficient 1 g(n) such that the running time of this algorithm is (g(n)), and briefly explain. public static int funkySum...

Study smarter with the SolutionInn App


