Use the data in the following table for three different aqueous solutions of (mathrm{CaCl}_{2}) to calculate the
Question:
Use the data in the following table for three different aqueous solutions of \(\mathrm{CaCl}_{2}\) to calculate the apparent value of the van't Hoff factor.
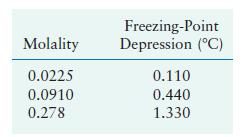
Transcribed Image Text:
Molality 0.0225 0.0910 0.278 Freezing-Point Depression (C) 0.110 0.440 1.330
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the apparent value of the vant Hoff factor i we can use the colligative property of fre...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the following exercises, you will use the data in the Solmaris Condominium Group database shown in Figures 1-21 through 1-25. (If you use a computer to complete these exercises, use a copy of the...
-
In the following exercises, you will use the data in the Solmaris Condominium Group database shown in Figures 1-21 through 1-25 in Chapter 1. (If you use a computer to complete these exercises, use a...
-
In the following exercises, you will use the data in the TAL Distributors database shown in Figure 2-1 in Chapter 2. (If you use a computer to complete these exercises, use a copy of the original TAL...
-
Sugar (C12H22O11) is a molecular compound that stays together inwater, while NaCl and MgSO4?7H2O are ionic compounds that dissociate into cations andanions as illustrated in the NaCl example below:...
-
The Pacific halibut fishery has been modeled by the differential equation dy/dt = ky (1 y/K) where y (t) is the biomass (the total mass of the members of the population) in kilograms at time...
-
The steel framework is used to support the reinforced stone concrete slab that is used for an office. The slab is 200 mm thick. Sketch the loading that acts along members BE and FED. Take a = 2 m, b...
-
Is this model useful for prediction? Why or why not? Use the = 0.05 level. Exercises 1115 refer to the following data set: x1 *2 x3 69.8 7.9 37.3 62.4 32.3 9.3 20.2 40.7 66.9 13.3 30.5 48.7 87.5...
-
How important was Ians knowledge of the billing procedures used within the steel industry in his being able to establish his business? Given his cash position when he opened his business, do you...
-
You are considering an investment in Fields and Struthers, Inc., and want to evaluate the firm s free cash flow. From the income statement, you see that Fields and Struthers earned an EBIT of $ 8 2...
-
The Tyndall effect is often used to distinguish between a colloidal suspension and a true solution. Explain.
-
Consider the following: What would happen to the level of liquid in the two arms if the semipermeable membrane separating the two liquids was permeable to the following? a. \(\mathrm{H}_{2}...
-
InfoSonics Corporation is a rapidly growing provider of wireless handsets and accessories in the United States and Latin America. The company distributes products for many important manufacturers,...
-
Bunga Raya Kuning Berhad is a Malaysian-based MNC that obtains 12 percent of its supplies from the U.K. manufacturers. Sixty-five percent of its revenues are from due to exports to U.K. where its...
-
To what extent can social conflict lead to positive social change, and what conditions are necessary for conflict to act as a catalyst for progress and transformation in society ?
-
The market is expected to return 15 percent next year and the risk-free rate is 7 percent. What is the expected rate of return on a stock with a beta of 1.3? The covariance of the market's returns...
-
Use the compound interest formula for continuous compounding to determine the accumulated balance after the stated period. 2) A $\$ 6958$ deposit in an account with an APR of $6.5 \%$ compounded...
-
3. In its natural state, a moist soil has a volume of 0.33 ft and weighs 39.33 lb. The oven- dried weight of the soil is 34.54 lb. The specific gravity of solids (Gs) is 2.67. W (%) W Y Wa (a)...
-
What are the differences between recourse, nonrecourse, and qualified nonrecourse liabilities? Which liabilities are considered at-risk?
-
Sue Deliveau opened a software consulting firm that immediately paid $2,000 for a computer. Was this event a transaction for the business?
-
Which would you expect to have a higher molar entropy at T = 0, single crystals of BF 3 or of COF 2 ? Why?
-
Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as heat to 0.820 mol Kr(g) at 298 K and 1.00 atm (a) At constant pressure; (b) At constant volume. Treat...
-
Dehydrated meals carried on camping trips are reconstituted with hot water. The energy used to heat the water is typically supplied by burning fuel in a camp stove. How much energy is required to...
-
Kim works for Priority Packaging in Alberta and is paid $2,519.79 semi-monthly. She contributes 4% of her gross earnings to a Registered Retirement Savings Plan each pay. Kim pays $23.00 semi-monthly...
-
The King Carpet Company has $3,110,000 in cash and a total of $11,430,000 in current assets. The firm's current liabilities equal $5,550,000 such that the firm's current ratio equals 2.1. The...
-
Matt Simpson owns and operates Quality Craft Rentals, which offers canoe rentals and shuttle service on the Nantahala River. Customers can rent canoes at one station, enter the river there, and exit...

Study smarter with the SolutionInn App


