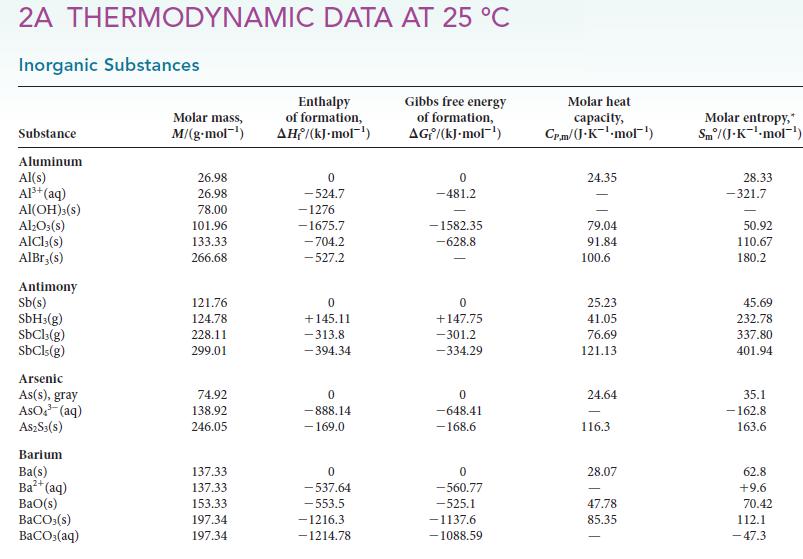
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of
Question:
Use the standard Gibbs free energies of formation in Appendix 2A to calculate ΔG° for each of the following reactions at 25 °C. Comment on the spontaneity of each reaction under standard conditions at 25 °C.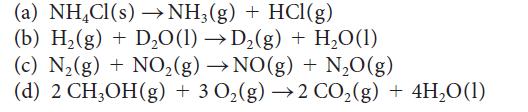
Transcribed Image Text:
(a) NHCl(s) NH(g) + HCl(g) (b) H(g) + DO(l) D(g) + HO(1) (c) N(g) + NO (g) NO(g) + NO(g) (d) 2 CHOH(g) + 3 0(g) 2 CO(g) + 4HO(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Use the relationship AG AG products AG reactants r a AGAGNH3 g AG HCl gAG NH4Cl s 1645 kJ mol9530 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Consider the exchange rate between South Korea and Costa Rica. Typically, exchange rates vary over time, sometimes quite dramatically. The scenarios present various changes that may affect the...
-
For the cylindrical capacitor of Problem 1.6c, evaluate the variational upper bound of Problem 1.17b with the naive trial function, 1() = (b )/(b a). Compare the variational result with the exact...
-
What ism-commerce? What is near field communication, and how does it facilitate mobile payments?
-
In the Earth reference frame, box 1 is approaching box 2 , which is initially at rest on a low-friction floor, with velocity \(v\). Box 1 has five times the inertia of box 2 . They collide...
-
Summary information from the financial statements of two companies competing in the same industry follows. Required 1. For both companies compute the (a) Current ratio, (b) Acid-test ratio, (c)...
-
31 The number of protons, electrons and neutrons in aluminium ion Al+ is Protons A. 27 B. 13 C. ABCD 32 32. D. 13 10 Electron 27 neutrons 14 14 14 10 14 17 14 The formula of the compound formed...
-
The following picture shows a molecular visualization of a system undergoing a spontaneous change. Account for the spontaneity of the process in terms of the entropy changes in the system and the...
-
Estimate the molar heat capacity (at constant volume) of sulfur dioxide gas. In addition to translational and rotational motion, there is vibrational motion. Each vibrational degree of freedom...
-
The soils in Problem 2.56 have the following Atterberg limits and natural water contents. Determine the PI and LI for each soil and comment on their general activity.?
-
Explain the difference between a pull and a push system, and the pros and cons of each model (principle 3).
-
In January, the interest rate is 5 percent and firms borrow $50 billion per month for investment projects. In February, the federal government doubles its monthly borrowing from $25 billion to $50...
-
Label each of the following scenarios as an example of a recognition lag, administrative lag, or operational lag. a. To fight a recession, Congress has passed a bill to increase infrastructure...
-
When bond prices go up, interest rates go . a. up b. down c. nowhere
-
What evidence would you ask to examine to determine if an organization is leveling the workload (principle 4)?
-
A city must decide whether to build a downtown parking garage (for up to 750 cars) and what rate to charge. It is considering two rates: a flat $1.50-per-hour rate or an all-day rate averaging $1 per...
-
A supermarket chain is interested in exploring the relationship between the sales of its store-brand canned vegetables (y), the amount spent on promotion of the vegetables in local newspapers (x1)...
-
Give the structure for each of the following aromatic hydrocarbons. a. o-ethyltoluene b. p -di- tert -butylbenzene c. m -diethylbenzene d. 1-phenyl-2-butene
-
Name each of the following alkenes or alkynes.
-
Name each of the following cyclic alkanes and indicate the formula of the compound.
-
The first production department in a process manufacturing system reports the following unit data. Beginning work in process inventory Units started and completed 33,600 units 50,400 units Units...
-
Carla Vista Corporation sells three different models of a mosquito "zapper." Model A12 sells for $59 and has unit variable costs of $41. Model B22 sells for $118 and has unit variable costs of $83....
-
Please discuss the fall protection processes involved with steel erection. There are a number of variations permitted during this work that is almost exclusive to this trade work. Please put...

Study smarter with the SolutionInn App


