Using only the periodic table, predict the most stable ion for (mathrm{Na}, mathrm{Mg}, mathrm{Al}, mathrm{S}, mathrm{Cl}, mathrm{K},
Question:
Using only the periodic table, predict the most stable ion for \(\mathrm{Na}, \mathrm{Mg}, \mathrm{Al}, \mathrm{S}, \mathrm{Cl}, \mathrm{K}, \mathrm{Ca}\), and \(\mathrm{Ga}\). Arrange these from largest to smallest radius and explain why the radius varies as it does. Compare your predictions with Fig. 13.8.
Figure 13.8
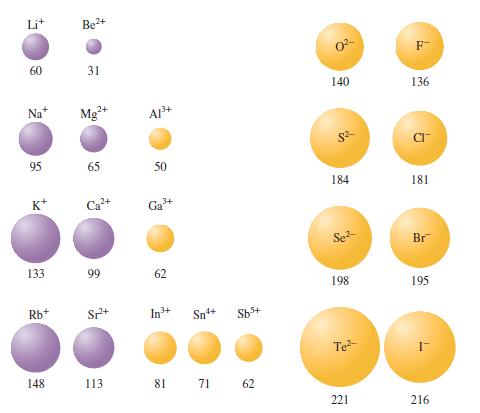
Transcribed Image Text:
60 Na* 95 K* 133 Rb+ Be+ 31 Mg+ 65 A1+ Sp+ 50 3+ Ga 99 62 In+ Sn4+ Sb+ 148 113 81 71 62 0- 140 $2 184 Se- 198 Te- 221 F- 136 CI- 181 Br 195 216
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Na Sodium Predicted most stable ion Na loses one electron to achieve a noble gas configuration Larger radius than Na Already provided Explanation The ...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the ionic compounds KF, NaCl, NaBr, and LiCl. (a) Use ionic radii (Figure 7.8) to estimate the cationanion distance for each compound. (b) Based on your answer to part (a), arrange these...
-
Graph f(x) - x, g(x) x +3 and (x) x5. Calculate the derivatives of f, g and h.
-
Using only the periodic table as your guide, select the most electronegative atom in each of the following sets: (a) Na, Mg, K, Ca; (b) P, S, As, Se; (c) Be, B, C, Si; (d) Zn, Ge, Ga, As.
-
Our model of pollution in this chapter assumed that emissions are a pure private bad, and that people have no ability to protect themselves from the adverse consequences of exposure. In reality,...
-
A particle of mass m is at r4est at the end of a spring (force constant = k) hanging from a fixed support. At t = 0, a constant downward force F is applied to the mass and acts for time t0. Show...
-
Find the expression for (v 2 x ) 1/2 , the rootmean-square value of v x , and the expression for the standard deviation of v x .
-
Reconsider the data from Problem 56. What is the capital recovery cost of Model 334A? Data from problem 56 Octavia Bakery is planning to purchase one of two ovens. The expected cash flows for each...
-
Nordham Corporation?s trial balance at December 31, 2012, is presented below. All 2012 transactions have been recorded except for the items described below. Unrecorded transactions 1. On January 1,...
-
While many parties were interested in acquiring MCI, the major players included Verizon and Qwest. U.S.-based Qwest is an integrated communications company that provides data, multimedia, and...
-
Which has the greater bond lengths: \(\mathrm{NO}_{2}{ }^{-}\)or \(\mathrm{NO}_{3}{ }^{-}\)? \(\mathrm{Ex}-\) plain.
-
Predict the trend in radius of the following ions: Be 2+ , Mg 2+ , Ca 2+ , and Sr 2+ .
-
Write down the meaning of each of the given equations. See Example 1. y = 2 sin 1 x Data from Example 1 (a) y = cos 1 x is read as y is the angle whose cosine is x. In this case, x = cos y. (b) y =...
-
A startled murder hornet jumps at a velocity of 1 8 m / s at an angle of 4 3 degrees above the horizontal. How far does she go if she jumps from a hill that is 5 m tall?
-
A crate having a mass of 500 kg is lifted vertically from rest with a uniform acceleration by a crane such that after 5 seconds it has a velocity of 8 m/s. Calculate the tension in the lifting cable.
-
On January 2, 2017, SilverCorp. bought a trademark from Lake Inc. for $150,000. An independent research company estimated that the remaining useful life of the trademark was 30 years. At this time,...
-
An impulse turbine with a single row wheel is to develop 99.3 kW, the blade speed being 150 m/sec. A mass of 2 kg of steam per second is to flow from the nozzles at a speed of 350 m/sec. The velocity...
-
Kwame makes $475,000 annually. He can deposit $23,000 per year into his NYC DCP account (retirement). Kwame currently has $13,000 in his NYC DCP account. He is 36 years old, wants to retire at 67....
-
Refer to the example in Appendix B. The numbers in Exhibit 5.21 for the fifth, sixth, and seventh units were given. Required Using the formula Y = aXb and the data given in the problem, verify the...
-
Express these numbers in standard notation. a. 2.87 10-8 b. 1.78 1011 c. 1.381 10-23
-
Predict the standard potential of each of the following galvanic cells: 3+ (a) Pt(s)| Fe+ (aq), Fe+ (aq)||Ag* (aq) Ag(s) (b) U(s) U+ aq||V+ (aq) V(s) 2+ (c) Sn(s) Sn+ (aq)||Sn4+ (aq),Sn+ (aq)|Pt(s)...
-
The molar solubility of silver sulfite, Ag 2 SO 3 , is 1.55 * 10 5 mol L 1 . What is the K sp of silver sulfite?
-
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: 2+ (a) Zn(s) Zn+ (aq)|| Au+ (aq)| Au(s) 3+ (b) Fe(s) | Fe+ (aq) || Fe+ (aq) |Fe(s)...
-
On your worksheet, fill in the values of A through N. A through F are denoted by red letters on the previous page and G through N are denoted by red letters on the flowchart below. What does this...
-
The tapered rod has a radius of r as shown below. Determine the average normal stress at the center of the rod: point B. = w (60+40x) lb/in. 3 in. = (2 ) in. T= B -3 in.- x
-
3. Consider a shaft mounted with frictionless bearings at its ends. For T = 400 Nm, TB =175 Nm, and Tc = 225 Nm, the maximum internal torque seen by the shaft is best given by T400 a. 800 Nm b. 225...

Study smarter with the SolutionInn App


