When lead(II) sulfide is treated with hydrogen peroxide, the possible products are either lead(II) sulfate or lead(IV)
Question:
When lead(II) sulfide is treated with hydrogen peroxide, the possible products are either lead(II) sulfate or lead(IV) oxide and sulfur dioxide.
(a) Write balanced equations for the two reactions.
(b) Use data from Appendix 2A to decide which possibility is more likely.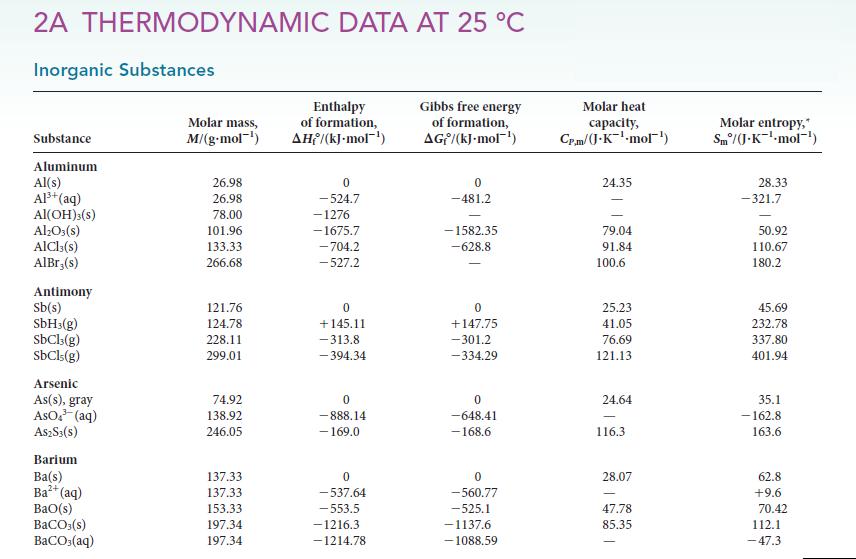
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(s) Al₂O3(s) AICI,(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls(g) Arsenic As(s), gray AsO4³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ.mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ-mol-¹) 0 -481.2 -1582.35 -628.8 0 +147,75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J-K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The two possible balanced equations for the reactions are as follows Equation 1 PbS 4HO PbSO 4HO Q ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
Write balanced equations for the following reactions: (a) Barium oxide with water, (b) Iron (II) oxide with perchloric acid, (c) Sulfur trioxide with water, (d) Carbon dioxide with aqueous sodium...
-
Write balanced equations for each of the following reactions. (a) When mercury(II) oxide is heated, it decomposes to form O2 and mercury metal. (b) When copper(II) nitrate is heated strongly, it...
-
Suppose you observe the following exchange rates: 1 = $1.50; 120 = $1.00. Calculate the euro-yuan exchange rate. Group of answer choices a. 80 = 1.00 b. 1 = 2.50 c. 133.33 = 1.00 d. 1.00 = 180
-
Draw a figure like Figure 2.1 to represent the following situation. a. A firm starts out with $10 million in cash. b. The rate of interest r is 10 percent. c. To maximize NPV the firm invests today...
-
Selected transactions for L. Takemoto, an interior decorating firm, in its first month of business, are shown below and on page 86. Jan. 2 Invested $15,000 cash in the business in exchange for common...
-
Let \(q(t)\) be the survival probability and let \(q^{-1}\) be its inverse function. Also, let \(U\) be a uniform random variable on \([0,1]\). For each realization \(u\), let \(\tau\) be chosen such...
-
Cruise Corporation had outstanding 100,000 shares of no-par common stock. On January 10, 2011, Dock Company purchased a block of these shares in the open market at $20 per share for long-term...
-
Kohl's Department Store and Target Corporation buy their clothing from Form Fitters Inc., a merchandising firm, that has budgeted\ its activity for December according to the following information:\...
-
The interhalogen IFx can be made only by indirect routes. For example, xenon difluoride gas can react with iodine gas to produce IF x and xenon gas. In one experiment, xenon difluoride is introduced...
-
The interhalogen ClF x has been used as a rocket fuel. It reacts with hydrazine to form the gases hydrogen fluoride, nitrogen,and chlorine. In one study of this reaction, ClF x gas is introduced into...
-
Show that the area of two other of the six nontrivial unitarity triangles of the CKM matrix are equal to half of the Jarlskog invariant, \(J\).
-
Calgary Paper Company produces paper for photocopiers. The company has developed standard overhead rates based on a monthly capacity of 64,000 direct-labor hours as follows: Standard costs per unit...
-
Question 3 Suppose that there are four risky assets whose expected returns E(r) and variance- covariance matrix (S) are shown in the spreadsheet below. We also consider the portfolio weights of two...
-
Question 4 i. Do you agree with the statement "The implied volatility of an option which has a given strike price and expiry date can vary over time"? Explain in detail. [5 marks] H. A bond has face...
-
From the Statement of Operations: (For the most recent year reported) Did gross profit increase or decrease over prior year? By how much? ($) By how much (%) Did income /(loss) from operations...
-
Data for M. Oriole, interior decorator, are presented as follows. Jan. 2 Invested $13,900 cash in business. 3 9 11 16 20 23 28 Purchased used car for $4,170 cash for use in business. Purchased...
-
Service personnel must be aware of the degree of social distance desired by their customers. Explain.
-
Medi-Exam Health Services, Inc. (MEHS), located in a major metropolitan area, provides annual physical screening examinations, including a routine physical, EKG, and blood and urine tests. MEUS's...
-
Methane belongs to the T d group. The reducible representation for the vibrational modes is reducible = A 1 + E + 2T 2 . a. Show that the A 1 and T 2 representations are orthogonal to each other and...
-
Use the 3 Ã 3 matrices for the C 2v group in Equation (27.2) to verify the associative property for the following successive operations: a. b. ,(6,C2) = (6,6-)C () = 6,(2) %3D
-
Use the logic diagram of Figure 27.2 to determine the point group for allene. Indicate your decision-making process as was done in the text for NH3 1. linear? 2. C n axis? 3. more than 1C n axis? 4....
-
Question 2. No-Arbitrage Determination of Forward Price [20%] The information of the forward price and stock price is provided below: Forward price F 0 $567 Stock/Spot Price S 0 $485 Maturity date of...
-
A bank offers an annual rate of 10% with monthly compounding if you invest $30,000 for 12 years. 1.1 Calculate the amount of money in the account by the end of the 12 years. 1.2 Calculate the annual...
-
General Motors issued 1 year note in the Euro-bond market that matures in 1 year. The bond has a par value of $100 and it will pay a 5.5% coupon. What is the investor's exposure to loss in the event...

Study smarter with the SolutionInn App


