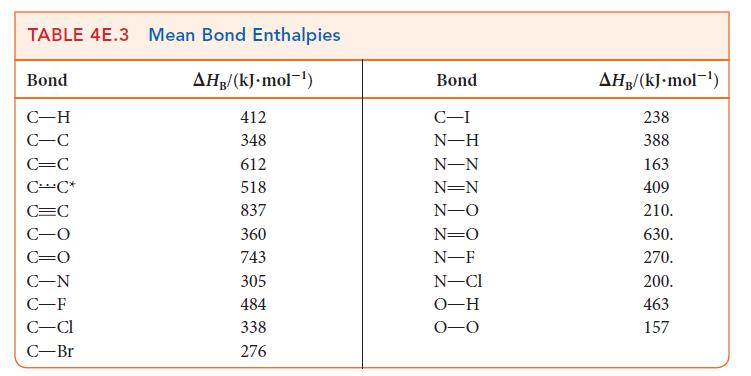
Write the balanced chemical equation for the fluorination of methane to difluoromethane. Use bond enthalpies (Tables 4E.2
Question:
Write the balanced chemical equation for the fluorination of methane to difluoromethane. Use bond enthalpies (Tables 4E.2 and 4E.3) to estimate the enthalpy of this reaction. The corresponding reaction using chlorine is much less exothermic. To what can this difference be attributed?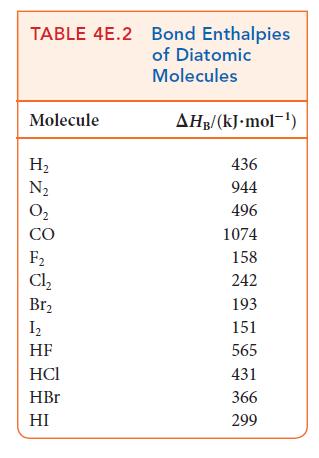
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





