You are working in a plant that produces copper for use in electrical circuits by electrolysis of
Question:
You are working in a plant that produces copper for use in electrical circuits by electrolysis of acidic solutions of CuSO4(aq). A customer has placed an order for a small quantity of high-purity copper, and you need to tell the customer how long it will take to produce the metal. How many hours are required to plate 25.00 g of copper metal from 1.00 m CuSO4(aq) by using a current of 3.00 A?
PLAN Use the second procedure in Toolbox 6O.1.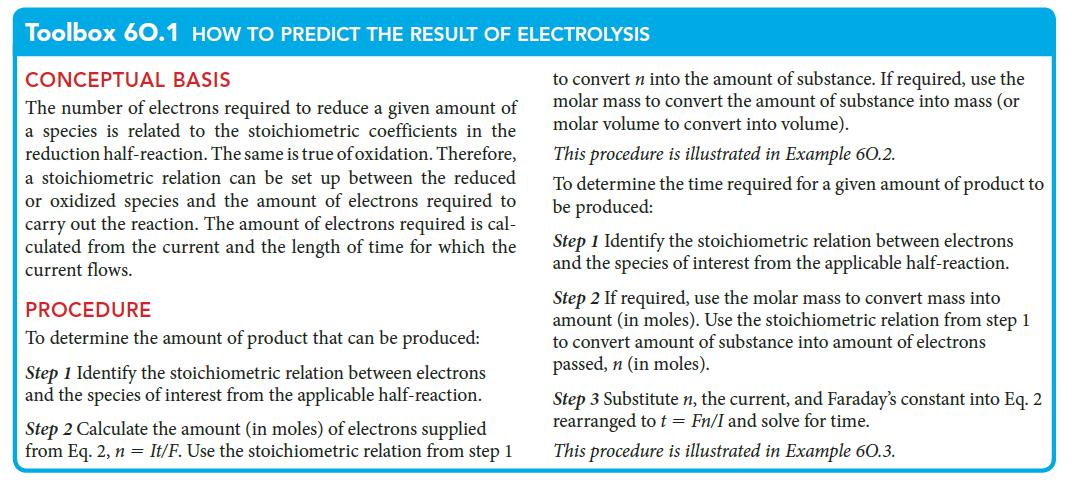
Transcribed Image Text:
Toolbox 60.1 HOW TO PREDICT THE RESULT OF ELECTROLYSIS CONCEPTUAL BASIS The number of electrons required to reduce a given amount of a species is related to the stoichiometric coefficients in the reduction half-reaction. The same is true of oxidation. Therefore, a stoichiometric relation can be set up between the reduced or oxidized species and the amount of electrons required to carry out the reaction. The amount of electrons required is cal- culated from the current and the length of time for which the current flows. PROCEDURE To determine the amount of product that can be produced: Step 1 Identify the stoichiometric relation between electrons and the species of interest from the applicable half-reaction. Step 2 Calculate the amount (in moles) of electrons supplied from Eq. 2, n = It/F. Use the stoichiometric relation from step 1 to convert n into the amount of substance. If required, use the molar mass to convert the amount of substance into mass (or molar volume to convert into volume). This procedure is illustrated in Example 60.2. To determine the time required for a given amount of product to be produced: Step 1 Identify the stoichiometric relation between electrons and the species of interest from the applicable half-reaction. Step 2 If required, use the molar mass to convert mass into amount (in moles). Use the stoichiometric relation from step 1 to convert amount of substance into amount of electrons passed, n (in moles). Step 3 Substitute n, the current, and Faraday's constant into Eq. 2 rearranged to t = Fn/I and solve for time. This procedure is illustrated in Example 60.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Step 1 Find the stoichiometric relation between electrons and the species of interest ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
1. You will be implementing the Generalized Arc Consistency (GAC) algorithm described in Chapter 4 of the textbook. In particular, you will be implementing the while loop shown in Figure 4.3:...
-
A customer has placed an order for 30,000 first-aid kits that must be delivered within 20 business days. The kits are to be assembled on the four-workstation line shown below and in kanban quantities...
-
Use hand calculations to fit the multiple linear regression model 1 y = β0 + β1x1 + β2x2 to the data set in DS 13.6.2. (a) Write down the vector of observed values...
-
Make versus buy, activity-based costing opportunity costs. (N. Melumad and S. Reichelstein, adapted) The Ace Company produces bicycles. This years expected production is 10,000 units. Currently, Ace...
-
You have just been hired as the director of operations for Reid Chocolates, a purveyor of exceptionally fine candies. Reid Chocolates has two kitchen layouts under consideration for its recipe making...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The total payroll of Rene Auber Company for September 2008 was $480,000, of which $110,000 is exempt from FICA tax because it represented amounts paid in excess of $97,500 to certain employees. The...
-
Consider three incomes: $35 000, $65 000, and $130 000. Determine the maximum RRSP contribution and the tax benefit at each income level. What would be an equivalent TFSA contribution? Next project...
-
Muscles produce lactic acid during exercise. Calculate the pH, pOH, and percentage deprotonation of the following aqueous solutions of lactic acid, CH 3 CH(OH)COOH: (a) 0.11 m; (b) 3.7 * 10 3 m; (c)...
-
Wine is a complex mixture of over 1000 compounds, many at very low concentrations. A large number of flavorful components are derivatives of phenol, C 6 H 5 OH, a weak acid. If you are an enologist...
-
Find the best approximation to a solution of the given system of equations. x + y - z = 2 -y + 2z = 6 3x + 2y - z = 11 -x + z = 0
-
For questions 2.A and 2.B below, use the data sets to draw a graph using the dynamic macro model. Year Fed Funds Rate Inflation Using CPI 2.0 Housing Starts Real GDP Growth 1.0 1.0 2.2 2.0 2.2 3.1...
-
a) Recall the problem from HW#3. The answer of this question is available on Canvas: Zixu Pec Co. (ZP) has expanded and now has both more refineries, and more oil fields in their supply chain. You...
-
1. Complete the required calculations on this worksheet in the boxes provided. Show your work for 2018 and 2019 as shown for 2020 that has been completed for you. Round all of your answers to 2...
-
1. 2. 3. 4. 5. 6. For every sales rep, list the sales rep's last name and the sales rep's first name in lower case letters. For every customer, list the customer's last name and the customer's first...
-
Find data with the following properties: The estimated regression function for the data has a positive slope It is steep for small values of the independent variable but less steep for large values...
-
REPCO performs warranty repair work for name-brand kitchen appliances. REPCO bills appliance manufacturers on a costplus basis. It has a job-costing system that computes the cost of each order by...
-
Which of the following is NOT a magnetic dipole when viewed from far away? a) A permanent bar magnet. b) Several circular loops of wire closely stacked together with the same current running in each...
-
Form the operator A 2 if A = x d/dx. Be sure to include an arbitrary function on which the operator acts.
-
Determine in each of the following cases if the function in the first column is an eigenfunction of the operator in the second column. If so, what is the eigenvalue? a. b. c. 1 cose sin e- sin e de...
-
Use a Fourier series expansion to express the function f (y) = y 2 , b ¤ y ¤ b, in the form Obtain d 0 and the first five pairs of coefficients c n and d n . cos f0) -d0+ , sin |+ dc...
-
Government claims to unpaid taxes Salary during last month owed to Mr. Key (not an officer) Administrative expenses Salary during last month owed to Ms. Rankin (not an officer) Unsecured accounts...
-
Gitano Products uses job-order costing and applies overhead cost to jobs based on direct materials used in production (not on the basis of raw materials purchased). Its predetermined overhead rate is...
-
! Required information [The following information applies to the questions displayed below.] Oslo Company prepared the following contribution format income statement based on a sales volume of 1,000...

Study smarter with the SolutionInn App


