A first-order reaction is taking place inside a porous catalyst. Assume dilute concentrations and neglect any variations
Question:
A first-order reaction is taking place inside a porous catalyst. Assume dilute concentrations and neglect any variations in the axial (x) direction.
a. Derive an equation for both the internal and overall effectiveness factors for the rectangular porous slab shown in Figure P15-9A.
b. Repeat part (a) for a cylindrical catalyst pellet where the reactants diffuse inward in the radial direction.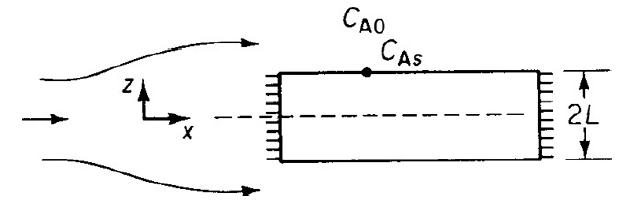
Flow over porous catalyst slab. A porous catalyst slab is shown in the form of a rectangle. The slab has a width of 2L along the z-axis and length is along x-axis. The direction of flow is also along the x axis. The concentration outside the slab is C subscript A0 and at the slab is C subscript As.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





