A mixed flow reactor (2 m 3 ) processes an aqueous feed (100 liter/min) containing reactant A
Question:
A mixed flow reactor (2 m3) processes an aqueous feed (100 liter/min) containing reactant A (CA0 = 100 mmol/liter). The reaction is reversible and represented by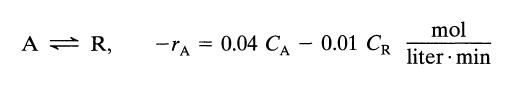
What is the equilibrium conversion and the actual conversion in the reactor?
Transcribed Image Text:
A = R, -TA 0.04 CA - 0.01 CR mol liter min
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
To determine the equilibrium conversion and actual conversion in the mixed flow reactor we need to consider the reversible reaction and use the given ...View the full answer

Answered By

Sarah Khan
My core expertise are:
-_ Finance
-_ Business
-_ Management
-_ Marketing Management
-_ Financial Management
-_ Corporate Finance
-_ HRM etc...
I have 7+ years of experience as an online tutor. I have hands-on experience in handling:
-_ Academic Papers
-_ Research Paper
-_ Dissertation Paper
-_ Case study analysis
-_ Research Proposals
-_ Business Plan
-_ Complexed financial calculations in excel
-_ Home Work Assistance
-_ PPT
-_ Thesis Paper
-_ Capstone Papers
-_ Essay Writing etc...
5.00+
91+ Reviews
92+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Your supervisor has just finished taking a college course in which she learned about flowcharting and data-flow diagrams. In the data-flow diagrams, there are four basic symbols that are used. Write...
-
A plug flow reactor (2 m 3 ) processes an aqueous feed (100 liter/min) containing reactant A (C A0 = 100 mmol/liter). This reaction is reversible and represented by First find the equilibrium...
-
How should we operate a mixed flow reactor so as to maximize the production of R? Separation and recycle of unused reactant is not practical. When aqueous A and aqueous B (C A0 = C B0 ) are brought...
-
Answer the Multple Choice Questions and the code for problem 6in the end PROBLEM 1: General UNIX 1. What is UNIX? a) an operating system b) a text editor c) programming language d) software program...
-
The limit order book for a security is as follows: The specialist receives the following, in order: Market order to sell 300 shares Limit order to buy 100 shares at 25.38 Limit order to buy 500...
-
Roche and Young, CAs, are preparing their service revenue (sales) budget for 2012. Their practice is divided into three departments: auditing, tax, and consulting. Billable hours for each department,...
-
In Example 2, the adult weighing 285 pounds decides to not participate in the study. What is the median weight of the remaining adults? Data from Example 2 Find the median of the weights listed in...
-
The comparative balance sheets of Nike, Inc. are presented here. Instructions(a) Prepare a horizontal analysis of the balance sheet data for Nike using 2006 as a base.(Show the amount of increase or...
-
A metal rod with 1 0 mm diameter is subjected to 9 kN tensile load. Calculate the resulting diameter of the rod after loading. Assume that the modulus of elasticity is 7 0 GPa, Poisson s ratio is 0 ....
-
A specific enzyme acts as catalyst in the fermentation of reactant A. At a given enzyme concentration in the aqueous feed stream (25 liter/min) find the volume of plug flow reactor needed for 95%...
-
The off gas from a boiling water nuclear power reactor contains a whole variety of radioactive trash, one of the most troublesome being Xe-133 (half life = 5.2 days). This off gas flows continuously...
-
Opinions are split about the Pumpkin Spice Latte (PSL). Yet every year over the last five years the consumption of pumpkin-flavored goods has increased (loseit.com). If we look at the numbers, it...
-
3. Consider a derivative that gives the buyer an option to receive the average of the past stock price values (including today's price) by paying. 100 dollars. The stock currently trades at S(0) =...
-
Mary borrowed $800from a bank for 3years and was charged simple interest. The total interest that she paid on the loan was $216.00. As a percentage, what was the annual interest rate of her loan?...
-
39. Use the following data and calculate the effort and duration required for every task, considering the following constraints: Task Design Write High Level DD Review High Level DD Level DD 1. Every...
-
Would the use of different logic gates be better in your electronics? Why or why not?
-
Does a callable bond benefit the issuer of a bond or the purchaser? Would the price of a callable bond be higher or lower than a non-callable bond, all else being equal? (3 marks) b. Does a...
-
According to the MR = MC rule, when the firm is producing at an output level where MR > MC, the firm should produce more. Explain.
-
Whats the difference between an ordinary annuity and an annuity due? What type of annuity is shown below? How would you change the time line to show the other type of annuity?
-
Use the data in Problem P11-4A for the following reaction. The elementary, irreversible, organic liquid-phase reaction A + B C is carried out in a flow reactor. An equal molar feed in A and B enters...
-
The elementary reversible liquid-phase reaction takes place in a CSTR with a heat exchanger. Pure A enters the reactor. (a) Derive an expression (or set of expressions) to calculate G(T) as a...
-
QBR Questions Before Reading. Research has shown (J. Exp. Psychol. Learn. Mem. Cogn., 40, 106114 (2014)) that if you ask a question of the material before reading the material you will have greater...
-
Explain the differences between capital expenditure and capital budgeting. Provide examples for each within the context of a hospital environment. Discuss how they relate to each othe?
-
Two companies in the transportation industry had the following information: Company 1 Company 2 Basic earnings per share $0.81 $0.69 Market price per share $11.97 $23.87 Dividends per share $0.512...
-
Chelsea has saved $20,000 for her son's education to date. He is 10 years old and she has 8 more years to save. If she invests $1,000 a year for the next 8 years, at the end of each year, how much...

Study smarter with the SolutionInn App


