a. The reaction A + B C occurs in the liquid phase. The proposed empirical rate
Question:
a. The reaction A + B → C occurs in the liquid phase. The proposed empirical rate law is −rA=A exp(−E/RT)CAαCBβ. Look at the values of s2 as a function of α. At what value of α is s2 minimized?
1. Vary β keeping A fixed and describe what you find.
2. Vary A keeping β fixed and describe what you find.
3. Suggest a rate law that minimizes s2.
b. Follow the Polymath tutorial to regress the data given in this example to fit the rate law
rCH4=kPCOαPH2β.
What is the difference in the correlation and sums of squares compared with those given in Example 7-4?
Example 7-4
The formation of methane from carbon monoxide and hydrogen using a nickel catalyst was studied by Pursley. The reaction
3H2 + CO → CH4 + H2O
J. A. Pursley, “An investigation of the reaction between carbon monoxide and hydrogen on a nickel catalyst above one atmosphere,” Ph.D. thesis, University of Michigan.
was carried out at 500°F in a differential reactor where the effluent concentration of methane was measured. The raw data is shown in Table E7-4.1.
TABLE E7-4.1 RAW DATA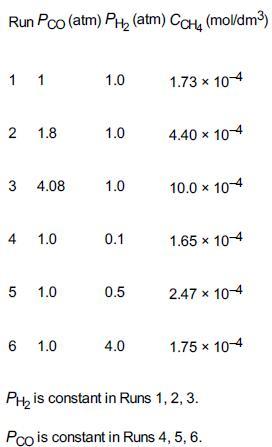
The exit volumetric flow rate from a differential packed bed containing 10 g of catalyst was maintained at S0 = 300 dm3/min for each run. The partial pressures of H2 and CO were determined at the entrance to the reactor, and the methane concentration was measured at the reactor exit. Determine the rate law and rate-law parameters. To better understand how we will determine the rate law and its parameters, we will break the solution into three parts, (a), (b), and (c), as shown below.
1. Relate the rate of reaction to the exit methane concentration. The reaction-rate law is assumed to be the product of a function of the partial pressure of CO, f(CO), and a function of the partial pressure of H2, g(H2), that is,
rCH4′=f(CO)⋅g(H2)
2. Determine the rate-law dependence on carbon monoxide, using the data generated in part (a). Assume that the functional dependence of rCH4′ on PCO is of the form
rCH4′∼PCOα
3. Generate a table of the reaction rate as a function of partial pressures of carbon monoxide and hydrogen, and then determine the rate-law dependence on H2.
Step by Step Answer:






