Choose five Chapter 10 i>clicker questions, pick the one that was the most challenging, and explain why.
Question:
Choose five Chapter 10 i>clicker questions, pick the one that was the most challenging, and explain why.
Chapter 5 Questions
1.How do the steps in the design of a CSTR differ from those of a CSTR or a PFR with pressure drop?
2.Go to the Web site (http://www.umich.edu/~elements/6e/05chap/iclicker_ch5_q1.html) and view at least five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explain why the question should not be on the next exam. In either case, explain your reasoning.
3.Read through all the problems at the end of this chapter. Make up and solve an original problem based on the material in this chapter.
(a) Use real data and reactions from the literature.
(b) Make up a reaction and data.
(c) Use an example from everyday life (e.g., making toast or cooking spaghetti). In preparing your original problem, first list the principles you want to get across and why the problem is important. Ask yourself how your example will be different from those in the text or lecture.
Other things for you to consider when choosing a problem are relevance, interest, impact of the solution, time required to obtain a solution, and degree of difficulty. Look through some of the journals for data, or to get some ideas for industrially important reactions, or for novel applications of reaction engineering principles (the environment, food processing, etc.). At the end of the problem and solution, describe the creative process used to generate the idea for the problem.
(d) Write a question based on the material in this chapter that requires critical thinking. Explain why your question requires critical thinking.
(e) Listen to the audios on the CRE Web site (www.umich.edu/~elements/6e/index.html) Lecture Notes, pick one, and describe how you might explain it differently.
4.If it takes 11 minutes to cook spaghetti in Ann Arbor, Michigan, and 14 minutes in Boulder, Colorado, how long would it take in Cuzco, Peru? Discuss ways to make the spaghetti more tasty. If you prefer to make a creative spaghetti dinner for family or friends rather than answering this question, that’s OK, too; you’ll get full credit—but only if you turn in your recipe and bring your instructor a taste.
5.Example 5-1.
(1) What would be the error in k if the batch reactor were only 80% filled with the same concentrations of reactants, instead of being completely filled as in the example?
(2) What generalizations can you draw from this example?
Example 5-1.
It is desired to design a CSTR to produce 200 million pounds of ethylene glycol per year by hydrolyzing ethylene oxide. However, before the design can be carried out, it is necessary to perform and analyze a batch-reactor experiment to determine the specific reaction-rate constant, k. Because the reaction will be carried out isothermally, the specific reaction rate will need to be determined only at the reaction temperature of the CSTR. At temperatures above 80°C, there is a significant byproduct formation, while at temperatures below 40°C, the reaction does not proceed at a significant rate; consequently, a temperature of 55°C has been chosen. Because water is present in excess, its concentration (55.5 mol/dm3) may be considered constant during the course of the reaction. The reaction is first-order in ethylene oxide.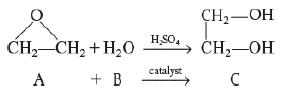
In the laboratory experiment, 500 mL of a 2 M solution (2 kmol/m3) of ethylene oxide (A) in water was mixed with 500 mL of water (B) containing 0.9 wt % sulfuric acid, which is a catalyst. The temperature was maintained at 55°C. The concentration of ethylene glycol (C) was recorded as a function of time (Table E5-1.1).
1. Derive an equation for the concentration of ethylene glycol as a function of time.
2. Rearrange the equation derived in (a) to obtain a linear plot of a function concentration versus time.
3. Using the data in Table E5-1.1, determine the specific reaction rate, k, at 55°C.
TABLE E5-1.1 CONCENTRATION–TIME DATA
Time (min) Concentration of Ethylene Glycol (C) (kmol/m3)*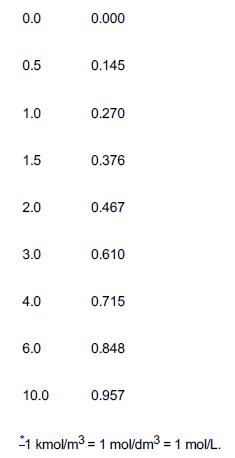


6.View the YouTube video titled CSTR to the tune of “It’s fun to stay at the YMCA” (https://www.youtube.com/watch? v=AkM67QsTq3E) made by the chemical reaction engineering students at the University of Alabama. You can also access it from the CRE Web site (http://www.umich.edu/~elements/6e/index.html); under “Additional Resources,” click on “Fun YouTube Videos.”
7.Are there steps in the Safety Analysis of the Incident that should be removed or added? If so, what are they?
8.Go to the five LearnChemE screencasts link for Chapter 5 (http://www.umich.edu/~elements/6e/05chap/learn-cheme-videos.html).
1. In the screencast of the PBR with pressure drop, is there a problem substituting for the volumetric flow rate when there is a change in the total number of moles, that is, δ ≠ 0?
2. View one of the other screencasts 5- to 6-minute video tutorials and list two of the most important points.
3. In the isothermal plug reactor screencast, how does the inert impact the reaction process?
9.AWFOS–S5 View the CSB video (https://www.youtube.com/watch?v=-_ZLQkn7X-k) and then research the conclusion as to whether or not the safety analysis of the incident is complete.
10.
(1) How would your profitumbers change in Table 5-4 if you used the following 2010 prices?
(2) Ethylene glycol $0.54/kg, ethylene $0.76/kg, ethylene oxide $1.17/kg, ethane $0.31/kg, sulfuric acid $0.10/kg (98 wgt %), and propylene glycol $1.70/kg.
(3) What pops out at you?
Table 5-4
1. Type in: https://us.vwr.com/store/search/searchMSDS.jsp
2. When the first screen appears, type in the chemical you need to find in the Keyword box and click on the Search button.
Example: Keyword Ethylene Glycol
3. The next page shows the list of Manufacturers (e.g., Alfa Aesar, MilliporeSigma) along with catalog number that provide the data on chemical you entered.
A screenshot displays the list of manufacturers and catalog numbers of Ethylene Glycol (99 percent, 2.5 kilograms) and Ethylene Glycol Anhydrous (4 liters). A link or button is provided to view SDS of the particular chemical. The catalog numbers shown for the chemicals are AAA11591-OE and EM-EX0566-4 respectively. The manufacturers shown for the chemicals are Alfa Aesar and MilliporeSigma respectively.
Click on “View SDS” under the SDS column for Alfa Aesarto see the material safety Data Sheet that will appear in a .pdf format
4. Scroll down for information you desire:
1. 1. Identification
2. 2. Hazard(s) Identification (GHS Pictographs)
3. 3. Composition/Information on Ingredients
4. 4. First Aid Measures
5. 5. Fire Fighting Measures
6. 6. Accidental Release Measures
7. 7. Handling and Storage
8. 8. Exposure Controls/Personal Protection
9. 9. Physical and Chemical Properties
10. 10. Stability and Reactivity
11. 11-16. Information such as Toxicological, Ecological, Disposal Considerations, Transport, Regulatory and Other Information
For ethylene oxide go to Cameo Chemicals (https://cameochemicals.noaa.gov/chemical/694).
Step by Step Answer:





