Circle the correct answer. (a) The elementary reversible isomerization of A to B was carried out in
Question:
Circle the correct answer.
(a) The elementary reversible isomerization of A to B was carried out in a packed-bed reactor. The following profiles were obtained: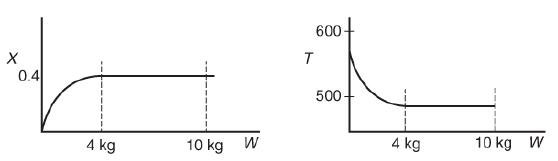
Two trend graph are shown. The horizontal axis of the first graph represents the weight W in Kg and the vertical axis of the first graph represents X. A trend curve is drawn on the graph from the origin and traces the path of the point 0.4 on the y-axis. Two dotted lines are drawn passing through the curve from the x-axis one for 4 kilograms and another for 10 kilograms. The horizontal axis of the second graph represents the weight W in Kilogram and the vertical axis of the second graph represents T and ranges from 500 to 600 in increments of 100. A trend curve is drawn on the graph from the point 570 on the y-axis and comes down to the point 500. Two dotted lines are drawn passing through the curve from the x-axis one for 4 kilograms and another for 10 kilograms. If the total entering volumetric flow rate remains constant, the addition of inerts to the feed stream will most likely
1) Increase conversion.
2) Decrease conversion.
3) Have no effect.
4) Insufficient information to tell
(b)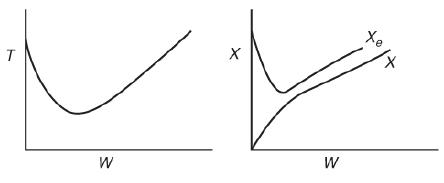
Two trend graph are shown. The horizontal axis of the first graph represents the weight W and the vertical axis of the first graph represents T. A trend curve is drawn on the graph from the a point on the y-axis. The horizontal axis of the second graph represents the weight W in Kg and the vertical axis of the second graph represents X. Two trend curves are drawn on the graph one representing X and another representing X Subscript e. Which of the following statements are true?
1) The above reaction could be adiabatic.
2) The above reaction could be exothermic with constant cooling temperature.
3) The above reaction could be endothermic with constant heating temperature.
4) The above reaction could be second order.
(c) The conversion is shown below as a function of catalyst weight down a PBR.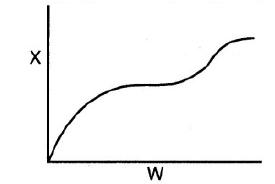
Which of the following statements are false?
1) The reaction could be first-order endothermic and carried out adiabatically.
2) The reaction could be first-order endothermic and reactor is heated along the length with Ta being constant.
3) The reaction could be second-order exothermic and cooled along the length of the reactor with Ta being constant.
4) The reaction could be second-order exothermic and carried out adiabatically.
5) The reaction could be irreversible.
To view more conceptual problems similar to (1)–(3) above go to http://www.umich.edu/~elements/6e/12chap/iclicker_ch12_q1.html.
Step by Step Answer:






