Consider the following elementary reactions: (a) One mole A and 3 moles B are rapidly mixed together.
Question:
Consider the following elementary reactions: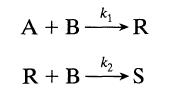
(a) One mole A and 3 moles B are rapidly mixed together. The reaction is very slow, allowing analysis of compositions at various times. When 2.2 moles B remain unreacted, 0.2 mole S is present in the mixture. What should be the composition of the mixture (A, B, R, and S) when the amount of S present is 0.6 mole?
(b) One mole A is added bit by bit with constant stirring to 1 mole B. Left overnight and then analyzed, 0.5 mole S is found. What can we say about k2/k1?
(c) One mole A and 1 mole B are thrown together and mixed in a flask. The reaction is very rapid and goes to completion before any rate measurements can be made. On analysis of the products of reaction 0.25 mole S is found to be present. What can we say about k2/k1?
Step by Step Answer:






