For the high gas velocity used assume that film diffusion does not offer any resistance to transfer
Question:
For the high gas velocity used assume that film diffusion does not offer any resistance to transfer and reaction. Reaction temperature = 900°C.![]()
Assuming that reaction proceeds by the shrinking-core model calculate the time needed for complete conversion of a particle and the relative resistance of ash layer diffusion during this operation.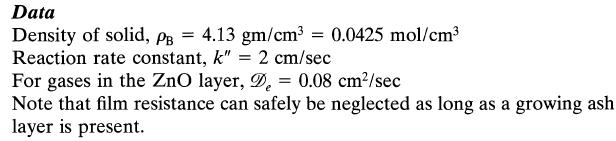
Transcribed Image Text:
2ZnS + 30₂2ZnO + 2SO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
SOLUTION Given Reaction 2ZnS 3O2 ZnO 2SO Temperature 900C Density of ZnS B 0...View the full answer

Answered By

Hassan Imtiaz
The following are details of my Professional Experience. Responsibilities Eight years of demanding teaching experience in the field of finance and business studies at Master’s Level. Completion of the given tasks within given time with quality and efficiency. Marketing professional with practical experience in and solid understanding of a diverse range of management applications, including market analysis, sales and marketing, team building and quality assurance. I have excellent skills to approach deal and sustain corporate clients / customers by demonstrating not only extraordinary communication and interpersonal skills but also high caliber presentation, negotiation and closing skills. Manage and follow up the day-to-day activities. Manage and co-ordinate the inventories. Fulfillment of all the tasks assigned.
The following are details of my Areas of Effectiveness. Finance 1. Corporate Finance 2. Advanced Corporate Finance 3. Management of Financial Institutions 4. International Financial Management 5. Investments 6. Fixed Income 7. Real Estate Investment 8. Entrepreneurial Finance 9. Derivatives 10. Alternative Investments 11. Portfolio Management 12. Financial Statement Analysis And Reporting (US GAAP & IFRS) 13. International Financial Markets 14. Public Finance 15. Personal finance 16. Real estate 17. Financial Planning Quantitative Analysis 1. Time Value Of Money 2. Statistics 3. Probability Distribution 4. Business Statistics 5. Statistical Theory and Methods Economics 1. Principles of Economics 2. Economic Theory 3. Microeconomic Principles 4. Macroeconomic Principles 5. International Monetary Economics 6. Money and Banking 7. Financial Economics 8. Population Economics 9. Behavioral Economics International Business 1. Ethics 2. Business Ethics 3. An introduction to business studies 4. Organization & Management 5. Legal Environment of Business 6. Information Systems in Organizations 7. Operations Management 8. Global Business Policies 9. Industrial Organization 10. Business Strategy 11. Information Management and Technology 12. Company Structure and Organizational Management Accounting & Auditing 1. Financial Accounting 2. Managerial Accounting 3. Accounting for strategy implementation 4. Financial accounting 5. Introduction to bookkeeping and accounting Marketing 1. Marketing Management 2. Professional Development Strategies 3. Business Communications 4. Business planning 5. Commerce & Technology Human resource management 1. General Management 2. Conflict management 3. Leadership 4. Organizational Leadership 5. Supply Chain Management 6. Law 7. Corporate Strategy Creative Writing 1. Analytical Reading & Writing Other Expertise 1. Risk Management 2. Entrepreneurship 3. Management science 4. Organizational behavior 5. Project management 6. Financial Analysis, Research & Companies Valuation 7. And any kind of Excel Queries
4.80+
150+ Reviews
230+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Calculate the time needed to burn to completion particles of graphite (R 0 = 5 mm, p B = 2.2 gm/cm 3 , k" = 20 cm/sec) in an 8% oxygen stream. For the high gas velocity used assume that film...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
c++. error: array must be initialized with a brace enclosed initializer main.cpp X 10 11 ii 12 13 14 4567 15 16 17 18 19 287288285 20 21 23 24 25 26 27 28 25285922223288 30 31 33 34 36 37 40 41 42 43...
-
Hydrotech Systems, Ltd., a New York corporation, agreed to sell wave-pool equipment to Oasis Waterpark, an amusement park in Palm Springs, California. Although Hydrotech was not licensed to install...
-
More U.S. law originates from one country than from any other. Which country? (a) France (b) England (c) Germany (d) Spain (e) Canada
-
The efficiency of a reversible machine is _____ 50 percent.
-
Identify specific fraud risk factors present during PwCs audits of the Lipper hedge funds. Explain how PwC should have responded to the fraud risk factors that you identified.
-
Distinguish the various methods used to engage and motivate employees for Baby Boomers. Explain these methods in details.
-
A batch of solids of uniform size is treated by gas in a uniform environment. Solid is converted to give a nonflaking product according to the shrinking-core model. Conversion is about $ for a...
-
A column packed with 5-cm polypropylene saddles (a = 55 m 2 /m 3 ) is being designed for the removal of chlorine from a gas stream (G = 100 mol/s m 2 , 2.36% Cl 2 ) by countercurrent contact with an...
-
On January 1, 2016, Maple Company invests $16,000 in Sprouts, Inc. stock. Sprouts pay Maple a $300 dividend on August 1, 2016. Maple sells the Sproutss stock on August 31, 2016, for $16,500. Assume...
-
Draw a flowchart to find if the user's input number is a prime number. Assume the number is an integer.
-
1. As explained in chapter one, accounting is a measurement and communication discipline based on rules referred to as generally accepted accounting (GAAP). Explain what are pros and cons of GAAP? ...
-
Give me feedback on this discussion please? analyzing the financial performance of the selected companies from the Data breach list, a comprehensive set of financial ratios has been employed to gain...
-
Tracy Corp. is considering Projects Meems and Eliza. The required return for each project is 11.4 percent. Calculate the NPV for each project. Which project should be accepted using NPV? Calculate...
-
convert following C-code to MIPS. You need to be explaining what you are typing in order to get FULL credit. int main() { int arr[]={5, 80, 123, 95, 882, 9, 11, -7, 87, 100, 24, 72, 86, 13, 61};...
-
Use the audit risk model as a framework to explain the following independent situations and to decide if the auditors conclusion is appropriate. Jan Morris, CPA, is finishing an audit of Night Time...
-
Huntingdon Capital Corp. is a competitor of Plazacorp and First Capital Realty. Huntingdon reported the following selected information (in millions):...
-
The following advertisement appeared in the Wall Street Journal on Thursday, February 9, 1995. ''There's nothing quite like the Seville Smart Lease. Seville SLS $0 down, $599 a monthj36 months" first...
-
After 15 Years of working for one employer, you transfer to a new job. During these years your employer contributed (that is, she diverted from your salary) $1500 each year to an account for your...
-
A finance company is using the following "Money by Mail" offer. Calculate the yearly nominal IRR received by the company if a customer chooses the loan of $2000 and accepts the credit insurance (Life...
-
If you Imagine yourself a senior analyst in the Office of Intelligence and Analysis within the Department of Homeland Security and your team has been tasked with compiling multiple sources of...
-
Determine how your selected organization could address the ethical issue you have identified through leveraging corporate social responsibility.
-
What measures have been taken to ensure the proper functioning of food commodity markets and their derivatives and facilitate timely access to market information, including on food reserves, to help...

Study smarter with the SolutionInn App


