Instead of using a trickle bed reactor for ethanol oxidation (see previous problem), let us consider using
Question:
Instead of using a trickle bed reactor for ethanol oxidation (see previous problem), let us consider using a slurry reactor.
For this type of unit
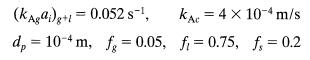
Take all flows and other values from the previous problem, and then find the expected fractional conversion of ethanol in this reactor.
Data from previous problem
Dilute aqueous ethanol (about 2-3%) is oxidized to acetic acid by the action of pure oxygen at 10 atm in a trickle bed reactor packed with palladium-alumina catalyst pellets and kept at 30°C. According to Sato et al., Proc. First Pacific Chem. Eng. Congress, Kyoto, p. 197,1972, the reaction proceeds as follows: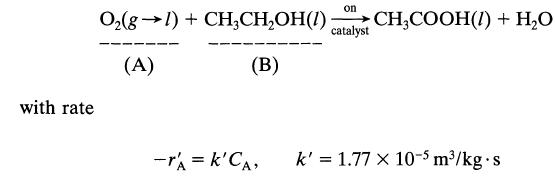
Find the fractional conversion of ethanol to acetic acid if gas and liquid are fed to the top of a reactor in the following system:

Step by Step Answer:






