List two similarities and two differences between the Safety Analysis of the Incident Algorithm and the BowTie
Question:
List two similarities and two differences between the Safety Analysis of the Incident Algorithm and the BowTie Diagram.
a. Example 6-1: Gas-Phase Reaction in a Microreactor Wolfram and Python
1. Use Wolfram and/or Python to Compare what happens when T and EA are each set at their maximum and minimum values (e.g., EAmax, Tmin, EAmax, Tmax), and then move the other slider and write a set of conclusions. Polymath
2. Compare Figure E6-1.1 profiles with those for a reversible reaction with KC = 0.02 mol/dm3 and describe the differences in the profiles.
3. How would your profiles change for the case of an irreversible reaction with pressure drop when αp = 99 × 103 dm–3 for each tube?
Example 6-1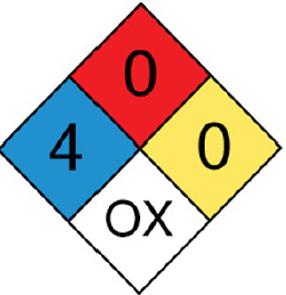
The blue diamond indicates a health hazard, the red diamond indicates a fire hazard, the white diamond indicates a specific hazard, and the red diamond indicates the reactivity. For chlorine, the health hazard is 4 which is of deadly, the fire hazard is 0, the reactivity is 0 that is stable, and the specific hazard is OX that indicates an oxidizer.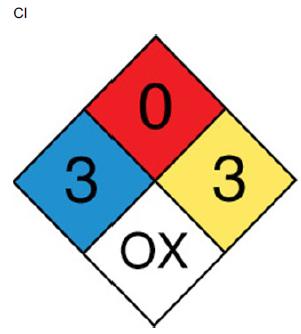
The blue diamond indicates a health hazard, the red diamond indicates a fire hazard, the white diamond indicates a specific hazard, and the red diamond indicates the reactivity. For Nitric oxide, the health hazard is 3 which is of extreme danger, the fire hazard is 0, the reactivity is 3 that indicates a violent chemical change, and the specific hazard is OX that indicates an oxidizer.
NO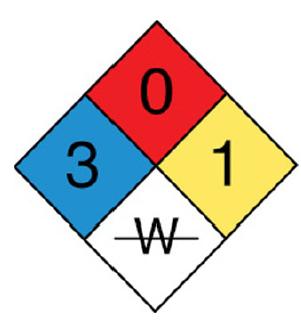
The blue diamond indicates a health hazard, the red diamond indicates a fire hazard, the white diamond indicates a specific hazard, and the red diamond indicates the reactivity. For Nitrosyl chloride, the health hazard is 3 which is of extreme danger, the fire hazard is 0, the reactivity is 1 that indicates unstable, and the specific hazard is W struck through that indicates no use of water.
NOCl
Nitrous oxide (NO) gas is used by a number of dentists on their patients (the author being one) to eliminate pain during drilling and tooth extraction. Nitrous oxide can be produced by the gas-phase second-order reaction.
2NOCl→2NO+Cl2
is to be carried out at 425°C and 1641 kPa (16.2 atm). Pure NOCl is to be fed, and the reaction follows an elementary rate law.2 It is desired to produce 20 tons of NO per year in a microreactor system using a bank of 10 microreactors in parallel. Each microreactor has 100 channels with each channel 0.2 mm square and 250 mm in length, giving a volume of a single channel to be 10–5dm3.
Additional Information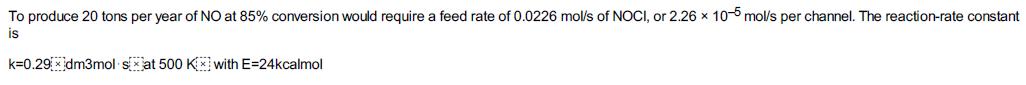
b. Example 6-2: Membrane Reactor
Wolfram and Python
A Stop and Smell the Roses Simulation. Vary the sliders to develop an intuitive feel of membrane reactors.
1. Starting with the default settings (all in proper default units) on the LEP simulation (i.e. , KC = 0.05, k = 0.7, CT0 = 0.2, and kC = 0.2), vary each parameter individually and describe what you find. Note and explain any maximum or minimum values of your plots down the length (i.e., volume = 500 dm3) of your reactor.
2. Repeat (i) but set KC at its maximum value and then vary k and kC, and describe what you find.
3. Write a set of conclusions from your experiments in (i) and (ii).
Polymath
4. Vary ratios of parameters such as (k/kC) and (kτCA0/KC) (note: τ = 400 min) and write a paragraph describing what you find. What ratio of parameters has the greatest effect on the conversion X = (FA0 – FA)/FA0?
5. Include pressure drop with α = 0.002 dm–3 and compare the conversion profiles for the two cases.
6. Write a summary paragraph of all the trends and your results.
7. Make up a question/problem on membrane reactors with a solution in which Wolfram must be used to obtain the answer.
Example 6-2
According to the Department of Energy (DOE), an energy saving of 10 trillion Btu per year could result from the use of catalytic membrane reactors as replacements for conventional reactors for dehydrogenation reactions such as the dehydrogenation of ethylbenzene to styrene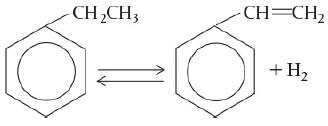
Ethylbenzene is a benzene with CH2CH3 attached to one carbon atom. After dehydrogenation, a double bond is formed for the ethane group (becomes ethene) attached and hydrogen molecule (H2) is released in the process. and of butane to butene:
The dehydrogenation of propane is another reaction that has proven successful with a membrane reactor.![]()
J. Membrane Sci., 77, 221 (1993). All the preceding elementary dehydrogenation reactions described above can be represented symbolically as A←→B+C and will take place on the catalyst side of an IMRCF. The equilibrium constant for this reaction is quite small at 227°C (e.g., KC = 0.05 mol/dm3). The membrane is permeable to B (e.g., H2) but not to A and C. Pure gaseous A enters the reactor at 8.2 atm and 227°C (CT0 = 0.2 mol/dm3) at a molar flow rate of 10 mol/min. The rate of diffusion of B out of the reactor per unit volume of reactor, RB, is proportional to the concentration of B (i.e., RB = kCCB).
1. Perform differential mole balances on A, B, and C to arrive at a set of coupled differential equations to solve.
2. Plot and analyze the molar flow rates of each species as a function of reactor volume.
3. Calculate the conversion of A at V = 500 dm3.
Additional information:
Even though this reaction is a gas–solid catalytic reaction, we will use the bulk catalyst density in order to write our balances in terms of reactor volume rather than catalyst weight (recall −rA=−rA′ρb and W = ρbV). For the bulk catalyst density of ρb = 1.5 g/cm3 and a 2-cm inside-diameter tube containing the catalyst pellets, the specific reaction rate, k, and the transport coefficient, kC, are k = 0.7 min–1 and kC = 0.2 min–1, respectively. As a first approximation, we will neglect pressure drop, that is, p = 1.
c. Example 6-2: Membrane Reactor Again
Polymath
Rework part (b) for the case when the reaction produces 3 mole of hydrogen
C6H12 ⇄ 3H2 + C6H6
You will need to make minor modifications to the LEP Polymath code on the Web site. All the parameters remain the same except the equilibrium constant, which is KC = 0.001 (moldm3)3.
d. Example 6-3: Isothermal Semibatch Reactor with a Second-Order Reaction
Wolfram and Python
1. Describe what happens as CB0, υ0, and V0 are varied one at a time between their maximum and minimum values. Explain why the variations from the base case look the way they do.
2. After using the slider to vary the parameter, write a set of conclusions.
Polymath
3. Redo this example problem assuming the reaction is reversible with KC = 0.1. Modify the Polymath code and compare with the irreversible case. (Only a couple of changes in the Poly-math program are necessary.)
Example 6-3
The production of methyl bromide is an irreversible liquid-phase reaction that follows an elementary rate law. The reaction
CNBr + CH3NH2 → CH3Br + NCNH2
is carried out isothermally in a semibatch reactor. An aqueous solution of methyl amine (B) at a concentration of CB0 = 0.025 mol/dm3 is to be fed at a volumetric rate of 0.05 dm3/s to an aqueous solution of bromine cyanide (A) contained in a glass-lined reactor.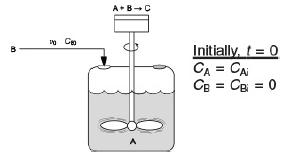
A container is given in which most of the container is filled with bromine cyanide. There are two openings on the top of the container in which one of the opening is closed and methyl amine is poured through the other opening. A rod like material with a fan at its end is fixed in the container and mixes the bromine cyanide and methyl amine. The combination of these two liquids then give methyl bromide.
The initial volume of liquid in the vat is to be 5 dm3 with a bromine-cyanide concentration of CA = CAi = 0.05 mol/dm3. The specific reaction-rate constant is k = 2.2dm3/s·mol Solve for the concentrations of bromine cyanide (A), methyl amine (B), methyl bromide (C), and cyanamide (D), and the rate of reaction as a function of time, and then analyze your results.
Step by Step Answer:






