One of the major reasons for engineoil degradation is the oxidation of the motor oil. To retard
Question:
One of the major reasons for engine–oil degradation is the oxidation of the motor oil. To retard the degradation process, most oils contain an antioxidant. Without an inhibitor to oxidation present, the suggested mechanism at low temperatures is
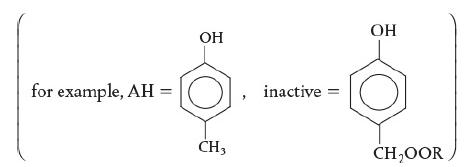
A possible example of antioxidants in a chemical reaction and an example of the inactive product formed in the end is shown. An example of antioxidants is shown as p-cresol or 4-methylphenol. It is a benzene compound with an alcohol group and a methyl group at positions 1 and 4. The end product formed is inactive. It is similar to p-cresol but instead of the methyl group attached at one end (4th position), the attached group or compound is CH2OOR.
a. Derive a rate law for the degradation of the motor oil in the absence of an antioxidant at low temperatures.
b. Derive a rate law for the rate of degradation of the motor oil in the presence of an antioxidant for low temperatures.
c. How would your answer to part (a) change if the radicals I· were produced at a constant rate in the engine and then found their way into the oil?
d. Sketch a reaction pathway diagram for both high and low temperatures, with and without antioxidant.
Step by Step Answer:






