The decomposition of reactant A at 400C for pressures between 1 and 10 atm follows a first-order
Question:
The decomposition of reactant A at 400°C for pressures between 1 and 10 atm follows a first-order rate law.
(a) Show that a mechanism similar to azomethane decomposition,
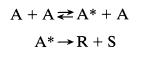
is consistent with the observed kinetics.
Different mechanisms can be proposed to explain first-order kinetics. To claim that this mechanism is correct in the face of the other alternatives requires additional evidence.
(b) For this purpose, what further experiments would you suggest we run and what results would you expect to find?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





