The decomposition of spartanol to wulfrene and CO2 is often carried out at high temperatures (J. Theor.
Question:
The decomposition of spartanol to wulfrene and CO2 is often carried out at high temperatures (J. Theor. Exp., 15, 15 (2014)). Consequently, the denominator of the catalytic rate law is easily approximated as unity, and the reaction is first order with an activation energy of 150 kJ/mol. Fortunately, the reaction is irreversible. Unfortunately, the catalyst over which the reaction occurs decays with time on stream. The following conversion–time data were obtained in a differential reactor: For T = 500 K: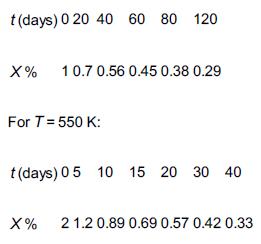 a. If the initial temperature of the catalyst is 480 K, determine the temperature–time trajectory to maintain a constant conversion.
a. If the initial temperature of the catalyst is 480 K, determine the temperature–time trajectory to maintain a constant conversion.
b. What is the catalyst lifetime?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





