The dehydrogenation of methylcyclohexane (M) to produce toluene (T) was carried out over a 0.3% Pt/ catalyst
Question:
The dehydrogenation of methylcyclohexane (M) to produce toluene (T) was carried out over a 0.3% Pt/ catalyst in a differential catalytic reactor. The reaction is carried out in the presence of hydrogen (H2) to avoid coking (J. Phys. Chem., 64, 1559 (1960)).
a. Describe how you would determine the model parameters for each of the following rate laws.
(1)−rM′=kPMαPH2β(3)−rM′=kPMPH2(1+KMPM)2(2)−rM′=kPM1+KMPM(4)−rM′=kPMPH21+KMPM+KH2PH2
Use the data in Table P10-16B below.
b. Which rate law best describes the data?
c. Suggest a mechanism and rate-limiting step consistent with the rate law you have chosen.
TABLE P10-16B DEHYDROGENATION OF METHYLCYCLOHEXANE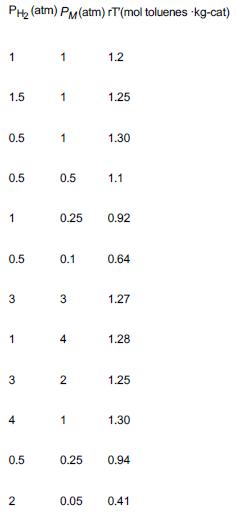
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





