The irreversible gas-phase reaction Acat B is carried out adiabatically over a packed bed of solid catalyst
Question:
The irreversible gas-phase reaction A→cat B is carried out adiabatically over a packed bed of solid catalyst particles. The reaction is first order in the concentration of A on the catalyst surface
−rAs′=k′CAs
The feed consists of 50% (mole) A and 50% inerts, and enters the bed at a temperature of 300 K. The entering volumetric flow rate is 10 dm3/s (i.e., 10000 cm3/s). The relationship between the Sherwood number and the Reynolds number is
Sh = 100 Re1/2
As a first approximation, one may neglect pressure drop. The entering concentration of A is 1.0 M. Calculate the catalyst weight necessary to achieve 60% conversion of A for
a. isothermal operation.
b. adiabatic operation.
Additional information: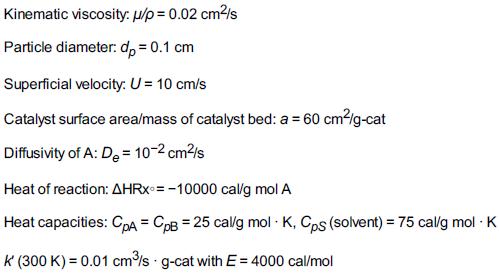
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





