The liquid-phase, dimer-quadmer series addition reaction 4 A 2A2 A4 can be written as 2AA2r1A=k1ACA2HRx1A=32.5kcalmol
Question:
The liquid-phase, dimer-quadmer series addition reaction 4 A → 2A2 → A4 can be written as
2A→A2−r1A=k1ACA2ΔHRx1A=−32.5kcalmol A2A2→A4−r2A2=k2A2CA22ΔHRx2A2=−27.5kcalmol A2
and is carried out in a 10-dm3 PFR. The mass flow rate through the heat exchanger surrounding the reactor is sufficiently large so that the ambient temperature of the exchanger is constant at Ta = 315 K. The reactants enter at a temperature T0, of 300 K. Pure A is fed to the rector at a volumetric flow rate of 50 dm3/s and a concentration of 2 mol/dm3.
(a) Plot, compare, and analyze the profiles FA, FA2, and FA4 down the length of the reactor up to 10 dm3.
(b) The desired product is A2 and it has been suggested that the current reactor may be too large. What reactor volume would you recommend to maximize FA2?
(c) What operating variables (e.g., T0, Ta) would you change and how would you change them to make the reactor volume as small as possible and to still maximize FA2? Note any opposing factors in maximum production of A2. The ambient temperature and the inlet temperature must be kept between 0°C and 177°C.
Additional information: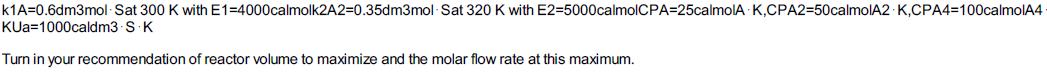
Step by Step Answer:






