The reaction A+BC is carried out adiabatically in a constant-volume batch reactor. The rate law is -rA=k1CA1/2CB1/2-k2CC
Question:
The reaction A+B→C is carried out adiabatically in a constant-volume batch reactor. The rate law is -rA=k1CA1/2CB1/2-k2CC Plot and analyze the conversion, temperature, and concentrations of the reacting species as a function of time.
Additional information: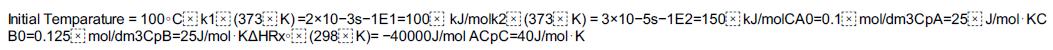
Transcribed Image Text:
Initial Temparature = 100°C k1x (373x K)=2x10-3s-1E1-100x kJ/molk2x (373x K) = 3x10-5s-1E2=150 kJ/molCA0=0.1 mol/dm3CpA=25J/mol KC B0=0.125 mol/dm3CpB=25J/mol KAHRX (298xK)= -40000J/mol ACpC=40J/mol K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Batch problem Mol balance Rate law Stoichiometry klT koexp dX Nao rav dt k2T koexp ra klCa2Cb2 k2Cc ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use the data and reaction in Problems P11-4A and P12-7B for the following reaction: A+B C+D (a) Plot and then analyze the conversion, Q r , Q g , and temperature profiles up to a PFR reactor volume...
-
Use the data in Problem P11-4A for the following reaction. The elementary, irreversible, organic liquid-phase reaction A + B C is carried out in a flow reactor. An equal molar feed in A and B enters...
-
A reaction is to be carried out in the packed-bed reactor shown in Figure P12-19C. PFR with heat exchange. The reactants enter the annular space between an outer insulated tube and an inner tube...
-
At what points are the function. y = x tan x 2 x + 1
-
Susan Calles lived with her four daughters, Amanda, age 11,Victoria, age 5, and Jenna and Jillian, age 3. In March 1998, Calles bought an Aim N Flame utility lighter, which she stored on the top...
-
Find the interest rates, or rates of return, on each of the following: a. You borrow $700 and promise to pay back $749 at the end of 1 year. b. You lend $700 and receive a promise to be paid $749 at...
-
Juliette Shulof Furs (JSF) was a New York corporation that had been in the fur-dealing business for 15 years. George Shulof, an officer of JSF, attended two auctions conducted by Finnish Fur Sales...
-
The following costs have been estimated for the activities in a project: (a) Develop a cost schedule based on earliest start times. (b) Develop a cost schedule based on latest start times. (c)...
-
Explain the relationship and the difference between online analytical processing systems and customer relationship management systems within a business intelligence program.?
-
You are buying a new car for $30,000 plus 5% taxes for a total cost of $31,500. You can pay cash for the entire amount by writing a check from your money market deposit account at the bank that...
-
The elementary irreversible liquid-phase reaction A+2BC is to be carried out in a semi batch reactor in which B is fed to A. The volume of A in the reactor is 10 dm 3 , the initial concentration of A...
-
The following is an excerpt from The Morning News, Wilmington, Delaware (August 3, 1977): Investigators sift through the debris from blast in quest for the cause [that destroyed the new nitrous oxide...
-
Go to www.irs.gov and search for IRS e-file security. List the facts that the IRS cites about why e-filing is secure. What about these practices makes the customers information secure? How could the...
-
a. Define what is Keynesian Economics and what is Neoliberalism. What is their main difference regarding the role of government in the market place/the economy and why it matters? b. Explain the...
-
Identify a characteristic of a defined contribution plan (DCP). Retirement income is determined by the employee's years of service and compensation. Employee contributions to a DCP do not reduce RRSP...
-
The most accurate statement based on the information provided is: "Lionel isn't required to sign his family returns because he does not charge for those, but he must sign any returns he prepares for...
-
Find (Y) for the following dataset: Y 5 3 4 8 2 6 7 6 1 2
-
A forensic scientist examines a hair with a microscope that has a 15x objective and a 5x eyepiece. The magnified hair has the same apparent size as a 2.0-cm-wide ribbon seen from a distance of 1.0 m....
-
The following accounts appear in an adjusted trial balance of Hampshire Consulting. Indicate whether each account would be reported in the (a) Current asset; (b) Property, plant, and equipment; (c)...
-
Chicago Company sold merchandise to a customer for $1,500 cash in a state with a 6% sales tax rate. The total amount of cash collected from the customer was $558. $600. $642. $636. Nevada Company...
-
Effect of pK a in the titration of weak acid with strong base. Use Equation 10-13 to compute and plot the family of curves at the left side of Figure 10-3. For a strong acid, choose a large K a ,...
-
Effect of concentration in the titration of weak acid with strong base. Use your spreadsheet from Problem 10-65 to prepare a family of titration curves for pK a = 6, with the following combinations...
-
Potassium ion in a 250.0 (0.1) mL water sample was precipitated with sodium tetraphenylborate: The precipitate was filtered, washed, dissolved in an organic solvent, and treated with excess Hg(EDTA)...
-
In process costing, factory rent and utilities are debited to the ________. Question content area bottom Part 1 A. Finished Goods Inventory account B. Manufacturing Overhead account C. WorkinProcess...
-
Complete this question by entering your answers in the tabs below. Required 1Required 2 Prepare a classified balance sheet at its December 31 year-end. (Hint: remember to include accumulated...
-
Data for the heights of 238 female volleyball players was collected. The average was 185cm and the variance was 36cm. A player in the sample had a height of 177cm. a. The height of 177cm is X...

Study smarter with the SolutionInn App


