Under the influence of oxidizing agents, hypophosphorous acid is transformed into phosphorous acid: The kinetics of this
Question:
Under the influence of oxidizing agents, hypophosphorous acid is transformed into phosphorous acid: 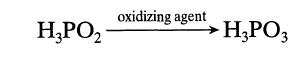
The kinetics of this transformation present the following features. At a low concentration of oxidizing agent,![]()
At a high concentration of oxidizing agent,![HPO = k[H+][HPO]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/0/5/7/9856517ca81bf6451696057983265.jpg)
To explain the observed kinetics, it has been postulated that, with hydrogen ions as catalyst, normal unreactive H3P02 is transformed reversibly into an active form, the nature of which is unknown. This intermediate then reacts with the oxidizing agent to give H3P02. Show that this scheme does explain the observed kinetics.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





