When a concentrated urea solution is stored it slowly condenses to biuret by the following elementary reaction:
Question:
When a concentrated urea solution is stored it slowly condenses to biuret by the following elementary reaction: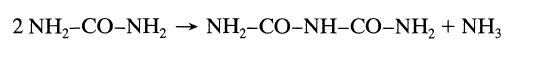
To study the rate of condensation a sample of urea (C = 20 mollliter) is stored at 100°C and after 7 hr 40 min we find that 1 mol% has turned into biuret. Find the rate equation for this condensation reaction. [Data from W. M. Butt, Pak. I. Ch. E., 1, 99 (1973).]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





