Xylene has three major isomers, m-xylene (A), o-xylene (B), and p-xylene (C). When m-xylene (A) is passed
Question:
Xylene has three major isomers, m-xylene (A), o-xylene (B), and p-xylene (C). When m-xylene (A) is passed over a Cryotite catalyst, the following elementary reactions are observed. The reaction to form p-xylene is irreversible: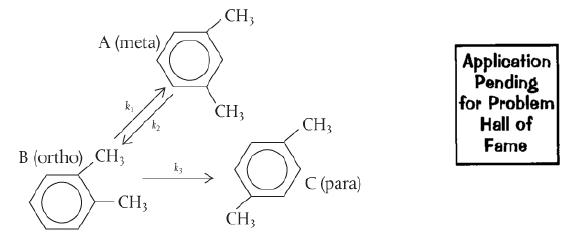
Xylene is a 6-membered aromatic compound with two alkyl groups. The ortho isomer shows the presence of alkyl group in positions 1 and 2 of the aromatic ring, which interchanges to take the meta form (alkyl groups in 1 and 3). The rate constants of the forward and backward reactions are k1 and k2. The conversion of ortho to para form (alkyl positions 1 and 4) is irreversible with rate constant k3.
The feed to the reactor is pure m-xylene (A). For a total feed rate of 2 mol/min and the reaction conditions below, plot the temperature and the molar flow rates of each species as a function of catalyst weight up to a weight of 100 kg.
(a) Plot the concentrations of each of xylenes down the length (i.e., V) of a PBR.
(b) Find the lowest concentration of o-xylene achieved in the reactor.
(c) Find the maximum concentration of o-xylene in the reactor.
(d) Repeat part (a) for a pure feed of o-xylene (B). What is the maximum concentration of meta-xylene and where does it occur in the reactor?
(e) Vary some of the system parameters and describe what you learn.
(f) What do you believe to be the point of this problem?
Additional information
Obtained from inviscid pericosity measurements. All heat capacities are virtually the same at 100 J/mol · K.![]()
Step by Step Answer:





