A buffer with a pH of 9.85 contains CH 3 NH 2 and CH 3 NH 3
Question:
A buffer with a pH of 9.85 contains CH3NH2 and CH3NH3Cl in water. What can you conclude about the relative concentrations of CH3NH2 and CH3NH3Cl in this buffer? For CH3NH2, pKb = 3.36.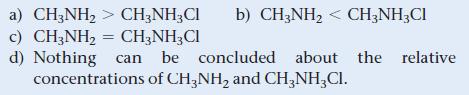
Transcribed Image Text:
a) CH3NH₂ > CH3NH3Cl b) CH3NH₂ < CH3NH3Cl c) CH3NH₂ = CH3NH₂Cl d) Nothing can be concluded about the relative concentrations of CH3NH₂ and CH3NH₂ Cl.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
b C...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A medical researcher wishes to determine the percentage of females who take vitamins. He wishes to be 99% confident that the estimate is within 2% percentage points of the true proportion. A recent...
-
What can you conclude about the relative risk of investing in the United States versus Japan from Figure 7.4?
-
In this problem, we consider the analysis of the combined information from both raters on the shoulder flexion (SF) scores in the posture measurement study. Thus, the questions below concern the data...
-
Use the Principle of Induction to prove the formula for all natural numbers \(n\). \(1+2+3+\cdots+n=\frac{n(n+1)}{2}\)
-
How are allocated joint costs treated when making a sell-or-process-further decision?
-
Confirm that the spherical harmonics (a) Yo,o' (b) Y2-1 and (c) Y3+3 satisfy the Schrdinger equation for a particle free to rotate in three dimensions, and find its energy and angular momentum in...
-
When completing a variables sampling plan, an auditor's evaluation is based on the relationship between and among a number of factors, including recorded account balance, estimated total population...
-
Decision Case 23-1 Movies Galore distributes DVDs to movie retailers, including dot.coms. Movies Galores top management meets monthly to evaluate the companys performance. Controller Allen Walsh...
-
(a) Record the above transactions. On January 1, 2024, Granger Corporation had 79,000 common shares, recorded at $545,100, and retained earnings of $1 million. During the year, the following...
-
Use the HendersonHasselbalch equation to calculate the pH of a buffer solution that is 0.50 M in NH 3 and 0.20 M in NH 4 Cl. For ammonia, pK b = 4.75.
-
A 1.0-L buffer solution contains 0.100 mol HC 2 H 3 O 2 and 0.100 mol NaC 2 H 3 O 2 . The value of Ka for HC 2 H 3 O 2 is 1.8 * 10 -5 . Because the initial amounts of acid and conjugate base are...
-
Happy Planet (http://www.happyplanet.com/), a Vancouver-based organic juice producer is an emerging player in the organic beverage market supplying all of Canada and some of the U.S. with organic...
-
As a result of the Coronavirus pandemic, Australian consumers have become increasingly pessimistic about their future job prospects and have decided to save more of every new dollar of income. If the...
-
Find and simplify f (x + h). f(x)=-2x-4x-7
-
1. What are the three main characteristics and uses of money. 2. Compare the National Banking Acts of 1863 with the chartering of the First and Second Banks of the United States. Specifically,...
-
1. Rollins College is an example of an 'institution' in the sense that Douglass North describes it. https://www.aeaweb.org/articles?id=10.1257/jep.5.1.97 Explain why and how, Rollins College can be...
-
Simplify the expression 18(x-3) 17x -10
-
Using the Wet Weekend Swim Park information presented, do the following tasks. Wet Weekend Swim Park sells individual and family tickets. With a ticket, each person receives a meal, three beverages,...
-
What is the ideal number of children to have? This question was asked on the Sullivan Statistics Survey I. Draw a dot plot of the variable Children from theSullivanStatsSurveyI data set at...
-
Using Fig. 10.87 , design a problem to help other students better understand the superposition theorem. R2 jXL ll V2 -jXc R1 V, (+1
-
Find v o for the circuit in Fig. 10.86 , assuming that i s (t) = 2 sin (2t) + 3 cos (4t) A. i,(t) 10 5 HE Vo rell
-
Determine V o and I o in the circuit of Fig. 10.80 using mesh analysis. J4 u . 2 3V. 2 10/-30 A + >I
-
Which is an example of an option to purchase additional goods or services that results in a material right? Loyalty points provided to a customer that acumulate to a right to free product. A promise...
-
Snyder Corporation is a small information systems consulting firm that specializes in helping companies implement sales management software. The market for Snyder's products is very competitive. To...
-
White Company has two departments, Cutting and Finishing. The company uses job-order costing and computes a predetermined overhead rate in each department. The Cutting Department bases its rate on...

Study smarter with the SolutionInn App


