A chemical reaction in which reactants A and B form the product C is studied in the
Question:
A chemical reaction in which reactants A and B form the product C is studied in the laboratory. The researcher carries out the reaction with differing relative amounts of reactants and measures the amount of product produced. Examine the given tabulated data from the experiment and answer the questions.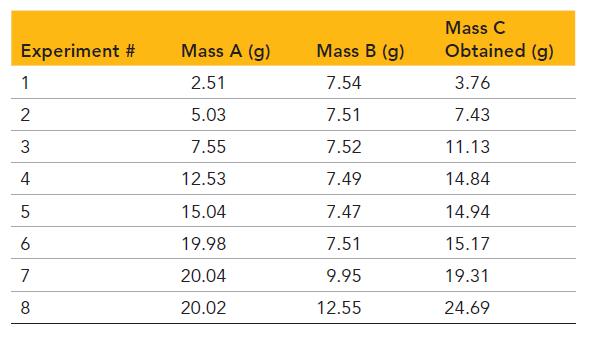
a. For which experiments is A the limiting reactant?
b. For which experiments is B the limiting reactant?
c. The molar mass of A is 50.0 g/mol, and the molar mass of B is 75.0 g/mol. What are the coeffecients of A and B in the balanced chemical equation?
d. For each of the experiments in which A is the limiting reactant, calculate the mass of B remaining after the reaction has gone to completion. Use the molar masses and coeffecients from part c.
e. The molar mass of C is 88.0 g/mol. What is the coefficient of C in the balanced chemical equation?
f. Calculate an average percent yield for the reaction.
Step by Step Answer:






