An alloy is a metallic mixture composed of two or more elements. As is the case in
Question:
An alloy is a metallic mixture composed of two or more elements. As is the case in all mixtures, the relative amounts of the elements in an alloy can vary. In some cases, the components of an alloy can have different crystal structures. For example, a nickel–chromium alloy consists of nickel, which has face-centered cubic structure, and chromium, which has a body centered cubic structure. Which structure does the alloy adopt?
It depends on the relative compositions. The phase diagrams for alloys such as these reveal the structure as a function of the alloy composition and temperature. For example, the nickel and chromium phase diagram from 700 °C to 1900 °C is shown here: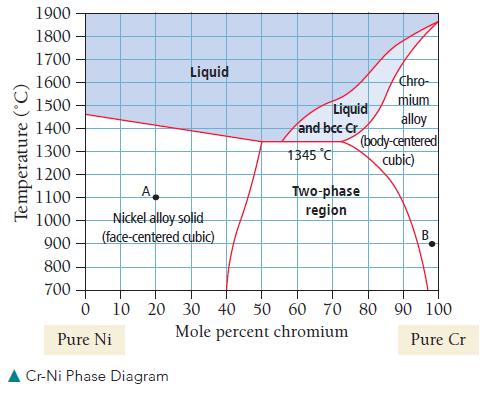
Notice that the diagram has two different solid phases: facecentered cubic and body-centered cubic. From pure nickel (0 mol % chromium) to about 40–50 mol % chromium, the structure is face-centered cubic. In this structure, Cr atoms substitute for Ni atoms in the face-centered cubic structure of nickel. However, when an amount of chromium beyond a certain percentage (which depends on temperature) is added, that structure is no longer stable. At the other end of the diagram, from pure chromium to about 75% chromium (depending on temperature), the structure is body-centered cubic, with nickel substituting into the body-centered cubic structure of the chromium. The region in between the two phases is called the two-phase region. At these compositions, the two phases (nickelrich face-centered cubic and chromium-rich body-centered cubic) exist together (the solution is not homogeneous in this region). Use the phase diagram to answer the following questions.
a. What is the relative composition of the mixture at point A?
At what temperature does a solid mixture having this composition melt?
b. Is it possible to have a homogeneous solid mixture that is 50% Ni and 50% Cr? If so, what crystalline structure would it have?
c. What is the relative composition at point B? What is the crystalline structure at point B?
d. At what temperature does the solid present at point B begin to melt?
Step by Step Answer:






